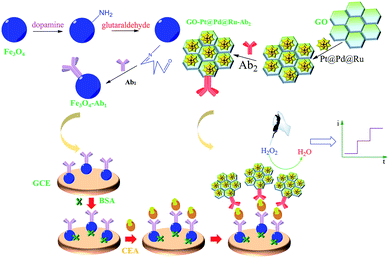
Ternary Pt@Pd@Ru nanodendrite-decorated graphene oxide for sensitive electrochemical immunoassy of CEA
Zonggang Mua,
Lei Jiaoa,
Qin Weia and
He Li*ab
aKey Laboratory of Chemical Sensing & Analysis in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China. E-mail: lihecd@gmail.com; Fax: +86 531 82765969; Tel: +86 130 82738030
bSchool of Biological Science and Technology, University of Jinan, Jinan 250022, China
First published on 26th April 2016
Abstract
Nobel metal nanoparticles have attracted intense attentions in biological immunoassay due to the inhereted good catalytic activity. In this study, a new kind of nanocomposite of ternary Pt@Pd@Ru nanodendrite-decorated graphene oxide (GO–Pt@Pd@Ru) was prepared as a signal label for ultrasensitive detection of carcinoembryonic antigen (CEA). With a sandwich type immunoassay, the established electrochemical immunosensor showed highly sensitive detection performance towards CEA in a linear range (0.1 pg mL−1 to 9 ng mL−1) with an ultralow detection limit of 0.05 pg mL−1. The enhancing detection performance could be attributed to: ternary Pt@Pd@Ru nanodendrites have exhibited superior electrochemical reduction activity towards H2O2, then GO–Pt@Pd@Ru can combine all generated electrochemical reduction current towards H2O2 by the decorated Pt@Pd@Ru nanodendrites, which can significantly amplify the detection signal.
1. Introduction
Sensitive and reliable detection of tumor biomarkers plays an important role in cancer diagnosis and therapy.1 The carcinoembryonic antigen (CEA) has been taken as a broad-spectrum tumor biomarker for clinical detection of some kinds of cancers, such as colon cancer, breast cancer and lung cancer.2 Therefore, there is an urgent desire to develop methods for sensitive detection of CEA. Currently, many immunoassay approaches, like magnetoimpedance immunosensor,3 Raman spectro-immunoassay,4 enzyme-linked immunosorbent assay,5 radioimmunoassay,6 chemiluminescence immunoassay7 and electrochemical immunoassay,8 have been used to detect CEA. Especially, electrochemical immunoassay has gained much more research interest owing to the advantages of portability, speedy analysis, cost effectiveness and high sensitivity.9 In order to enhance the sensitivity of electrochemical immunosensor, a variety of nanomaterials, such as graphene,10 carbon nanotubes,11 metal nanoparticles,12 were used achieving signal amplification for the detection of protein biomarkers. Among these nanomaterials, metal nanoparticles especially noble metal nano-alloy containing bimetallic and trimetallic nano-alloy attached considerable attentions.13 Compared with monometallic nanoparticles, nano-alloy presented higher catalytic performance.14–16 Herein, we demonstrated an electrochemical immunosensor using ternary Pt@Pd@Ru nanodendrite-decorated graphene oxide (GO–Pt@Pd@Ru) for sensitive detection of CEA. Ternary Pt@Pd@Ru nanodendrites have exhibited superior electrochemical reduction activity towards H2O2 because Pt@Pd@Ru nanodendrites provide many catalytically active sites for catalytic reactions. Therefore, the new nanocomposite of GO–Pt@Pd@Ru can merge all generated electrochemical reduction current towards H2O2 by loaded Pt@Pd@Ru nanodendrites and play as signal labels for electrochemical immunoassay of CEA, which can significantly improve the sensitivity of the developed electrochemical immunosensor.2. Experimental
2.1 Materials and apparatus
Graphene oxide (GO) was prepared by modified Hummer's method.17 Dopamine hydrochloride, potassium hexachloroplatinate (K2PtCl6) disodium tetrachloropalladate (Na2PdCl4) ruthenium chloride (RuCl3) and nano-iron oxide (Fe3O4) were purchased from Aladdin-industrial-corporation. Ascorbic acid (AA) and bovine serum albumin (BSA) and Pluronic F127 were bought from Sigma Company. Carcinoembryonic antigen (CEA) and CEA antibody were purchased from Shanghai E. Star Biotechnology Co., Ltd. (Shanghai, China). Phosphate buffered solutions (PBS) were prepared by mixing 0.067 mol L−1 Na2HPO4 and 0.067 mol L−1 KH2PO4 stock solution. Ultrapure water was used throughout the experiments.All electrochemical measurements were performed on a CHI760E electrochemical workstation (Chenhua Instrument Shanghai Co., Ltd., China). Transmission electron microscopy (TEM) images were recorded with a Philips CM200 UT (Field Emission Instruments, USA). Conventional three-electrode system was used for all electrochemical measurements: a glassy carbon electrode (GCE, 4 mm in diameter) as the working electrode, a platinum wire electrode as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode.
2.2 Synthesis of amino functionalization of Fe3O4 (NH2-Fe3O4) and conjugated Ab1
10 mg mL−1 of Fe3O4 was dispersed in 100 mL of dopamine hydrochloride solution (0.5 M). After that, this mixture was sonicated for 1 h and the product (NH2-Fe3O4) was washed with ultrapure water three times collected by magnet. 2 mg of NH2-Fe3O4 was dispersed in 1 mL PBS (pH 7.4) after adding 1 mL 0.25% glutaraldehyde and reacting for 12 h. Then, the mixture was separated by magnet. Afterwards, the product was shocked for 12 h after adding 1 mL of 200 ng mL−1 Ab1. Then, the solution was centrifuged to remove the unbonded antibody and then the product was redispersed in 1 mL PBS (pH 7.4) for electrochemical detection.2.3 Synthesis of GO–Pt@Pd@Ru nanocomposites
Pt@Pd@Ru nanodendrites were prepared following the previous work.18 Thereafter, 2.0 mg of Pd@Pt@Ru nanodendrites were added into 5 mL of GO (2 mg mL−1) solution and shocked for 12 h. Then, the resulted GO–Pt@Pd@Ru nanocomposites were collected after centrifugation and washed with ultrapure water for three times. Finally, the nanocomposites were dried under vacuum. Pt@Pd and Pt@Ru nanoparticles were prepared by the similar procedure of Pt@Pd@Ru, but there was no precursor solution of RuCl3 and Na2PdCl4, respectively.2.4 Synthesis of GO–Pt@Pd@Ru conjugated Ab2 as labels
2.0 mg of GO–Pt@Pd@Ru were dispersed in 2 mL of Ab2 (100 μg mL−1). The mixture was shocked at 4 °C for 12 h and washed with PBS (pH 7.4) in consecutive centrifugation. The final product (GO–Pt@Pd@Ru-Ab2) were redispersed in 1 mL of PBS (pH 7.4) and kept at 4 °C until used.2.5 Fabrication of immunosensor
The fabrication process of electrochemical immunosensor can be illustrated as Scheme 1. A GCE was polished with alumina slurry to obtain a mirror-like surface and dried at temperature. 6.0 μL of Fe3O4-Ab1 (1.5 mg mL−1) was dropped on the surface of GCE and left to dry naturally at room temperature. Then 3.0 μL of 1%wt BSA was put onto the electrode surface to block nonspecific active sites. Afterwards, the electrode was washing with PBS (pH 7.4) to remove the excess BSA and then 6.0 μL of CEA with different concentrations was added onto the electrode surface for one hour. Thereafter 6.0 μL of GO–Pt@Pd@Ru-Ab2 was added onto the electrode surface for one more hour, and the electrode was washed thoroughly with PBS (pH 7.4) for electrochemical detection.3. Results and discussion
3.1 Characterization of Fe3O4, GO–Pt@Pd@Ru and Pt@Pd@Ru
As shown in Fig. 1A, Fe3O4 nanoparticles exhibited irregular lamellar structure and its average diameter was 200 nm approximately. It was clearly observed that Pt@Pd@Ru had dendritic structure which could provide many catalytic active sites and present higher catalytic performance (Fig. 1B). It was obviously seen that Pt@Pd@Ru nanoparticles were uniformly decorated on the surface of GO, shown in Fig. 1C, indicating that GO–Pt@Pd@Ru were synthesized successfully.As revealed in Fig. 2, it was cyclic voltammetry (CV) curves of bare GCE (black curve) and the electrode modified with 1.5 mg mL−1 of NH2-Fe3O4 (red curve) in 0.1 mol L−1 KCl solution containing 5.0 mM Fe(CN)63−. It was evident that the CV of bare GCE and NH2-Fe3O4 modified electrode were both shown a well-defined reversible redox behavior of [Fe(CN)6]3−/4−. And the peak current of NH2-Fe3O4 modified electrode was higher than that of bare electrode, indicating that NH2-Fe3O4 modified electrode had better conductivity, which could improve the sensitivity of electrochemical immunosensor.
 | ||
| Fig. 2 Cyclic voltammetry curves of bare GCE (black) and the electrode modified with 1.5 mg mL−1 of NH2-Fe3O4 (red) in 0.1 mol L−1 KCl solution containing 5.0 mM Fe(CN)63−. | ||
Compared with the CV of bare GCE (curve a) in pH 7.4 of PBS, it could be seen that the CV of GO–Pt@Pd@Ru modified electrode (curve b) was shown a wider range, indicating GO–Pt@Pd@Ru presents good conductivity (Fig. 3A). Additionally, it could be seen that a reduction peak of H2O2 was at −0.3 V (curve c) with relative high reduction current. Fig. 3B presented the i–t curves of the electrodes modified by the same concentration of Pt@Ru (a), Pt@Pd (b), Pt@Pd@Ru (c) and GO–Pt@Pd@Ru (d) in pH 7.4 PBS with 5 mM H2O2 at −0.3 V. Obviously, GO–Pt@Pd@Ru showed the best electrochemical reduction performance towards H2O2. As a result, we established the sensitive electrochemical immunosensor for CEA detection using high catalytic GO–Pt@Pd@Ru as signal labels.
3.2 Characterization of the assembly procedure of immunosensor
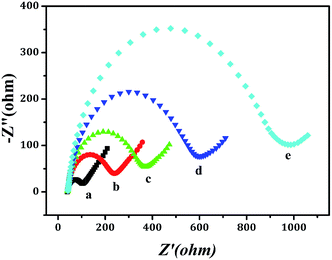
The assembly procedure of electrode was characterized by electrochemical impedance spectroscopy (EIS). The impedance spectra consist of a semicircle portion corresponding to the electron-transfer limited process and a linear portion representing the diffusion-limited process and the semicircle diameter was presented electron-transfer resistance.13 As shown in Fig. 4, it could be seen that the resistance of GCE (curve a) was the smallest one. After modifying Ab1-NH2-Fe3O4 (curve b), the resistance of electrode was increased obviously. The reason was that antibody as a kind of protein could hinder electro transfer. Similarly, because protein molecules could hinder electron transfer, the corresponding resistances (curve c, d and e) would increase sequentially when the electrode was following modified by BSA, CEA and GO–Pt@Pd@Ru-Ab2, which also indicated that the electrode was well-modified. In conclusion, the electrochemical immunosensor was established successfully.3.3 Optimizing conditions
In order to achieve good performance of the electrochemical immunosensor, experimental conditions of concentration of Fe3O4, concentration of GO–Pt@Pd@Ru and pH were optimized. It is clear to see that the current value reaches the maximum at the 1.5 mg mL−1 of Fe3O4 as shown in Fig. 5A (black line). Similarly, the maximum current response of the immunosensor occurred at 2.0 mg mL−1 of GO–Pt@Pd@Ru, pH 7.4 of PBS and 60 minutes of incubation time, seen from Fig. 5A (red line), Fig. 5B and C, respectively. Thus, 1.5 mg mL−1 of Fe3O4, 2.0 mg mL−1 GO–Pt@Pd@Ru and pH 7.4 of PBS were selected as the optimized conditions for the fabricated electrochemical immunosensor.3.4 Analytical performance
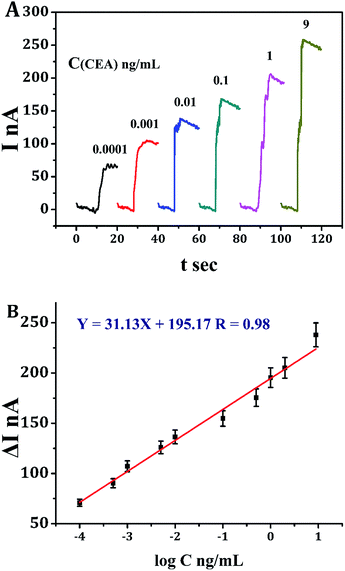
Under the optimal conditions, the immunosensor was fabricated for the detection of CEA. The current responses towards electrochemical reduction of H2O2 at −0.3 V were increased proportionally with the increasing concentrations of CEA, shown in Fig. 6A. Accordingly, a calibration curve towards the current responses for different concentrations of CEA was built: ΔI = 195.17 + 31.131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, R2 = 0.9836 (Fig. 6B). It is satisfied to see that the electrochemical immunosensor presented a broad linear range of 0.1 pg mL−1 to 9 ng mL−1 with an ultralow detection limit of 0.05 pg mL−1 (S/N = 3). As shown in Table 1, this method presented more sensitive performance towards CEA detection comparing with other reported methods.
C, R2 = 0.9836 (Fig. 6B). It is satisfied to see that the electrochemical immunosensor presented a broad linear range of 0.1 pg mL−1 to 9 ng mL−1 with an ultralow detection limit of 0.05 pg mL−1 (S/N = 3). As shown in Table 1, this method presented more sensitive performance towards CEA detection comparing with other reported methods.
3.5 Selectivity, stability and reproducibility
To investigate the selectivity of the developed electrochemical immunosensor, interference study was conducted using BSA, alpha-fetal protein (AFP), glucose and vitamin C (Vc) as interfering substances. 0.1 ng mL−1 of CEA adding 100 ng mL−1 of individual interfering substance were tested by the immunosensor, respectively. According to the results shown in Fig. 7A, the relative standard deviation (RSD) of current response with or without interfering substances was within 3%, indicating that the selectivity of the immunosensor was fairly good.To evaluate the stability of the immunosensor, the immunosensor was periodically measured the current response during the storage at 4 °C (Fig. 7B). Even after four weeks, the immunosensor could generate around 90% of the initial current. This result proved that the immunosensor had good stability.
To check the reproducibility of the immunosensor, 0.1 ng mL−1 of CEA was detected by a series of five electrodes (Fig. 7C). The measured RSD was within 4%, which means good reproducibility for the developed immunosensor.
In order to demonstrate the feasibility of the electrochemical immunosensor for clinical diagnosis, we measured the recovery of different concentrations of CEA by standard addition methods. As shown in Table 2, the recovery was within the range of 98.0–102%, revealed that the immunosensor could be applied into clinical analysis of CEA.
| Sample | Added (ng mL−1) | Found (ng mL−1) | Recovery (%) |
|---|---|---|---|
| 1 | 0.5 | 0.51 ± 0.02 | 102.0 |
| 2 | 1.0 | 0.98 ± 0.05 | 98.0 |
| 3 | 1.5 | 1.52 ± 0.1 | 101.3 |
| 4 | 2.0 | 2.04 ± 0.3 | 102.0 |
| 5 | 2.5 | 2.49 ± 0.4 | 99.6 |
4. Conclusions
In summary, Pt@Pd@Ru nanodendrites-decorated graphene oxide as a new kind of nanocomposite has been taken as signal label for enhancing sensitive detection of CEA. The excellent performance of the fabricated immunosensor could be contributed to: ternary Pt@Pd@Ru nanodendrites exhibited superior electrochemical reduction activity towards H2O2, and then Pt@Pd@Ru nanodendrite-decorated graphene oxide integrated all generated electrochemical reduction current towards H2O2 from each decorated Pt@Pd@Ru nanodendrite, which can significantly enhance the detection signal.Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (No. 21245007 and 81000976) and Postdoctoral Science Foundation of China (No. 2012M521295) for the financial support.Notes and references
- Y. Wang, Z. Ju, B. Cao, X. Gao, Y. Zhu, P. Qiu, H. Xu, P. Pan, H. Bao, L. Wang and C. Mao, ACS Nano, 2015, 9, 4475 CrossRef CAS PubMed.
- T. Letilovic, R. Vrhovac, S. Verstovsek, B. Jaksik and A. Ferrajoli, Cancer, 2006, 107, 925 CrossRef CAS PubMed.
- T. Wang, L. Guo, C. Lei and Y. Zhou, RSC Adv., 2015, 5, 51330–51336 RSC.
- M. Li, J. W. Kang, S. Sukumar, R. R. Dasari and I. Barman, Chem. Sci., 2015, 6, 3906 RSC.
- W. Zhou, J. Su, Y. Chai, R. Yuan and Y. Xiang, Biosens. Bioelectron., 2014, 53, 494 CrossRef CAS PubMed.
- E. Rubery, J. Doran and R. Thompson, Eur. J. Cancer Clin. Oncol., 1982, 18, 951 CrossRef CAS PubMed.
- J. S. Lee, H.-A. Joung, M.-G. Kim and C. B. Park, ACS Nano, 2012, 6, 2978 CrossRef CAS PubMed.
- D. Wu, H. Ma, Y. Zhang, H. Jia, T. Yan and Q. Wei, ACS Appl. Mater. Interfaces, 2015, 7, 18786 CAS.
- A. Chen and S. Chatterjee, Chem. Soc. Rev., 2013, 42, 5425 RSC.
- J. Liu, G. Lin, C. Xiao, Y. Xue, A. Yang, H. Ren, W. Lu, H. Zhao, X. Li and Z. Yuan, Biosens. Bioelectron., 2015, 71, 82 CrossRef CAS PubMed.
- R. Malhotra, V. Patel, J. P. Vaqué, J. S. Gutkind and J. F. Rusling, Anal. Chem., 2010, 82, 3118 CrossRef CAS PubMed.
- Y. Wang, H. Ma, X. Wang, X. Pang, D. Wu, B. Du and Q. Wei, Biosens. Bioelectron., 2015, 74, 59 CrossRef CAS PubMed.
- Q. Wei, Y. Zhao, B. Du, D. Wu, Y. Cai, K. Mao, H. Li and C. Xu, Adv. Funct. Mater., 2011, 21, 4193 CrossRef CAS.
- F. Nosheen, Z. Zhang, J. Zhuang and X. Wang, Nanoscale, 2013, 5, 3660 RSC.
- L. Wang and Y. Yamauchi, J. Am. Chem. Soc., 2013, 135, 16762 CrossRef CAS PubMed.
- Y. Wu, D. Wang, X. Chen, G. Zhou, R. Yu and Y. Li, J. Am. Chem. Soc., 2013, 135, 12220 CrossRef CAS PubMed.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Liu and J. M. Tour, ACS Nano, 2010, 4, 4806 CrossRef CAS PubMed.
- K. Eid, V. Malgras, P. He, K. Wang, A. Aldalbahi, S. M. Alshehri, Y. Yamauchi and L. Wang, RSC Adv., 2015, 5, 31147 RSC.
- Z. Wang, N. Liu, F. Feng and Z. Ma, Biosens. Bioelectron., 2015, 70, 98 CrossRef CAS PubMed.
- J. Miao, X. Wang, L. Lu, P. Zhu, C. Mao, H. Zhao, Y. Song and J. Shen, Biosens. Bioelectron., 2014, 58, 9 CrossRef CAS PubMed.
- X. Wang, C. Chu, L. Shen, W. Deng, M. Yan, S. Ge, J. Yu and X. Song, Sens. Actuators, B, 2015, 206, 30 CrossRef CAS.
- G. Sun, Y. Ding, C. Ma, Y. Zhang, S. Ge, J. Yu and X. Song, Electrochim. Acta, 2014, 147, 650 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |