A photoswitchable diarylethene heterodimer for use as a multifunctional logic gate†
Qi Ai and
Kwang-Hyun Ahn*
Department of Applied Chemistry, Kyung Hee University, Yongin 446-701, Republic of Korea. E-mail: khahn@khu.ac.kr; Fax: +82-31-202-7337; Tel: +82-31-201-2447
First published on 27th April 2016
Abstract
A novel multifunctional photochromic molecule, BTF–BTH, was synthesized via covalently linking two photoswitchable fluorescent diarylethenes BTFO4 and BTHTO, and the structure was characterized using NMR and MS analysis. The photophysical properties of four photoisomeric states, BTFo–BTHo, BTFc–BTHc, BTFc–BTHo and BTFo–BTHc, were studied. Interconversions among these states were readily achieved using light irradiation and the addition of Cu2+. Interestingly, a fluorescence energy transfer phenomenon was observed for BTFc–BTHc. Based on these results, logic gates, including OR, keypad lock, INHIBIT, AND, and 1:2 demultiplexer, were designed and successfully demonstrated using BTF–BTH.
Introduction
Recently, the simulation of electronic logic gates and circuits at the molecular level using individual molecules has attracted much attention.1,2 As a basic binary unit, photochromic compounds that can be interconverted between ‘off’ and ‘on’ states with different spectroscopic properties, representing ‘0’ and ‘1’ of a digital code, offer the possibility of establishing simple logic gates and more complex devices.3–8 Among photochromic compounds, diarylethenes are one of the most attractive families due to their promising fatigue resistance, efficient photo-isomerization, rapid response, and thermally irreversible properties.9,10 Because of these advantages, various applications of diarylethenes have been reported, including use as molecular logic systems by using multi-diarylethene molecules to store more data in a single entity, which overcomes the 0/1 barrier of a single diarylethene molecule.11–14In general, a molecular logic system uses an absorption or emission change as output in response to a variation of inputs, such as light irradiation or the presence of chemicals.15–23 Interestingly, oxidation or reduction can also be used as an additional input for the system with diarylethenes because electrochromic reactions accompanying cyclization or cycloreversion through electrochemical redox processes have been reported for many diarylethenes.24–27
The electrochromic ring-opening reaction can also rapidly take place in the presence of a catalytic amount of one-electron oxidizing reagents such as Cu2+.28 Compared with chemical ions that effect switching through binding to a ligand attached to diarylethenes, this unique electrochromic behavior offers a valuable additional means of accessing the two switching states and can be exploitable for molecular switches.29
In recent years, we reported two photoswitchable “turn-on” fluorescence diarylethenes, 1,2-bis(2-methyl-1-benzothiophene-1,1-dioxide-3-yl)perfluoro-cyclopentene (BTFO4)30–32 and 2,3-bis(2-methylbenzo[b]thiophen-3-yl)-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-one (BTHTO).33,34 Interestingly, as shown in Fig. 1, the emission spectrum of BTFO4c overlaps with the absorption spectrum of BTHTOc such that it might lead to fluorescence energy transfer from BTFO4c to BTHTOc when the two molecules are together in the same system. Furthermore, BTHTOc was rapidly transformed to BTHTOo in the presence of Cu2+, which acts as a one electron oxidant for BTHTOc.35 Based on these photochromic and electrochromic phenomena, we believed we could design a logic gate system using BTFO4 and BTHTO. Thus, we covalently connected the two diarylethenes to prepare a heterodimer BTF–BTH (Fig. 2). Because of energy transfer between BTFO4c and BTHTOc, and the electrochromic property of BTHTO with Cu2+, the heterodimer was able to perform the functions of OR,36 keypad lock,37,38 INHIBIT,39 and AND40 gates, as well as a 1:2 demultiplexer41 using light and the one-electron oxidant Cu(ClO4)2 as inputs and light as outputs. Herein, we report the synthesis and photophysical properties of the heterodimer as well as its various logic gate properties.
Experimental
Materials and characterization
All reagents and spectroscopic grade solvents were purchased from Aldrich. 1H and 13C NMR spectra were obtained using a Jeol JNM-AL300 spectrometer at 300 MHz and 75 MHz in CDCl3, respectively, with tetramethylsilane as an internal reference. HRMS spectra were obtained with a Jeol JMS-700 spectrometer. Absorption spectra were recorded on a Shimadzu UV-3100 spectrophotometer in spectroscopic grade ethyl acetate. Fluorescence spectra were collected in spectroscopic grade ethyl acetate on a Fluoro Max-2 spectrophotometer equipped with a 150 W ozone-free xenon lamp. UV and visible irradiations were performed with standard lamps used for visualizing TLC plates (VL6L; 312 nm, 8 MW cm−2) and a 100 W tungsten lamp.Synthesis of BTF–BTH
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 6 (0.27 g, 81%) as a white powder; mp: 250.5 °C; 1H NMR (CDCl3, 300 MHz) δ (ppm): 7.76–7.67 (m, 2H), 7.60–7.56 (m, 1.5H), 7.46–7.39 (m, 1.5H), 7.16–7.10 (m, 2H), 4.77 and 4.70 (s × 2, 2H), 2.19 and 2.05 (s × 2, 6H); 13C NMR (CDCl3, 75 MHz) δ (ppm): 144.90, 144.69, 143.95, 143.72, 143.04, 135.36, 135.06, 133.89, 133.72, 131.57, 131.31, 130.86, 130.71, 129.50, 129.39, 128.47, 120.38, 123.87, 123.75, 122.75, 122.58, 122.42, 120.83, 120.68, 115.17, 63.64, 30.94, 8.89; HRMS (FAB+): m/z calcd for C24H16F6O5S2 [M + H]+: 563.0422; found: 563.0430.
1) to give 6 (0.27 g, 81%) as a white powder; mp: 250.5 °C; 1H NMR (CDCl3, 300 MHz) δ (ppm): 7.76–7.67 (m, 2H), 7.60–7.56 (m, 1.5H), 7.46–7.39 (m, 1.5H), 7.16–7.10 (m, 2H), 4.77 and 4.70 (s × 2, 2H), 2.19 and 2.05 (s × 2, 6H); 13C NMR (CDCl3, 75 MHz) δ (ppm): 144.90, 144.69, 143.95, 143.72, 143.04, 135.36, 135.06, 133.89, 133.72, 131.57, 131.31, 130.86, 130.71, 129.50, 129.39, 128.47, 120.38, 123.87, 123.75, 122.75, 122.58, 122.42, 120.83, 120.68, 115.17, 63.64, 30.94, 8.89; HRMS (FAB+): m/z calcd for C24H16F6O5S2 [M + H]+: 563.0422; found: 563.0430.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give BTF–BTH (0.085 g, 70%) as a reddish powder; mp: 167.5–168.1 °C; 1H NMR (CDCl3, 300 MHz) δ (ppm): 8.34 and 8.32 (s × 2, 1H), 7.73–7.55 (m, 7H), 7.40–7.28 (m, 3H), 7.25–7.10 (m, 3H), 5.20 and 5.13 (s × 2, 2H), 3.48–3.37 (m, 6H), 2.89–2.81 (m, 4H), 2.42–1.80 (m, 14H); 13C NMR (CDCl3, 75 MHz) δ (ppm): 197.21, 144.07, 139.83, 137.90, 137.44, 133.86, 133.22, 133.16, 131.63, 130.84, 129.38, 127.82, 124.42, 124.11, 123.46, 122.98, 122.89, 122.76, 122.64, 122.52, 122.33, 122.16, 122.08, 121.98, 121.77, 64.50, 39.37, 39.26, 33.23, 31.92, 29.81, 29.70, 29.66, 29.36, 28.21, 15.41, 15.37, 15.18, 15.02, 8.90, 8.80; HRMS (FAB+): m/z calcd for C53H36F6O8S6 [M + H]+: 1107.0717; found: 1107.0721.
1) to give BTF–BTH (0.085 g, 70%) as a reddish powder; mp: 167.5–168.1 °C; 1H NMR (CDCl3, 300 MHz) δ (ppm): 8.34 and 8.32 (s × 2, 1H), 7.73–7.55 (m, 7H), 7.40–7.28 (m, 3H), 7.25–7.10 (m, 3H), 5.20 and 5.13 (s × 2, 2H), 3.48–3.37 (m, 6H), 2.89–2.81 (m, 4H), 2.42–1.80 (m, 14H); 13C NMR (CDCl3, 75 MHz) δ (ppm): 197.21, 144.07, 139.83, 137.90, 137.44, 133.86, 133.22, 133.16, 131.63, 130.84, 129.38, 127.82, 124.42, 124.11, 123.46, 122.98, 122.89, 122.76, 122.64, 122.52, 122.33, 122.16, 122.08, 121.98, 121.77, 64.50, 39.37, 39.26, 33.23, 31.92, 29.81, 29.70, 29.66, 29.36, 28.21, 15.41, 15.37, 15.18, 15.02, 8.90, 8.80; HRMS (FAB+): m/z calcd for C53H36F6O8S6 [M + H]+: 1107.0717; found: 1107.0721.Results and discussion
The structure of 2
Since the structure of BTHTO is asymmetric, acylation with succinic anhydride can give attachment at either the b or f positions of BTHTO, which leads to compound 2 or 2′, respectively (Scheme 1). To confirm the structure of 2, we synthesized disubstituted compounds 5 and 7 (ESI†) and compared their NMR spectra with that of 2 (Fig. 3). The aromatic region in the NMR of 2 provides valuable information about the structure. Two singlet peaks at 8.25 and 8.36 ppm correspond to the anti-parallel (ap) and parallel (p) conformers for Ha of 2 or He of compound 2′ because their combined intensity represents a single proton. The doublet at 7.87 ppm (J = 9 Hz) is expected to be either Hc of 2 or Hg of 2′. The NMR of disubstituted compound 5 showed two doublets at 7.94 and 7.86 ppm that may correspond to Hg and Hc of 5, respectively, because of the deshielding effect of the carbonyl group on Hc. Based on this analysis, the doublet at 7.87 ppm of 2 can be assigned as Hc instead of Hg of 2′. Furthermore, the NMR of compound 7 has a doublet at 7.86 ppm, which confirmed the structure of 2.Photochromism and electrochromism of the heterodimer
BTF–BTH can exist in four different isomeric states as shown in Scheme 2. Interconversion between these states could be readily induced by irradiation with light. Scheme 2 displays the solution color and fluorescence of the four photoisomeric structures of the heterodimer in ethyl acetate (1.0 × 10−5 M) induced by irradiation with various wavelengths of light. Upon irradiation of the BTFo–BTHo solution with 312 nm light, new absorption bands at 400 and 542 nm appeared, which from comparison with the absorption spectra of BTFO4c and BTHTOc may indicate the formation of BTFc–BTHc. In addition, irradiation of BTFc–BTHc with 540 nm light could only produce the BTFc–BTHo state because the BTHc moiety has a strong absorption and the BTFc moiety has no absorption at 540 nm. Upon irradiation of the heterodimer solution with 378 nm light for 1 h, BTFo–BTHo was isomerized mainly into BTFo–BTHc. Since the cyclization quantum yields of both BTFo and BTHo moieties are expected to be similar based on those of BTFO4 and BTHTO,30,33 the selective photo-cyclization may be caused by a much higher absorption at that wavelength (Fig. 4a). Notably, the BTFo–BTHo state can be reset from any state with visible light. Fig. 4 demonstrates that it is possible to obtain the four photoisomers mentioned above and that the spectra of the heterodimer in its various forms are very similar to linear combinations of the spectra of the model compounds 2 and 4, shown in Fig. 4a. Thus, linking the two diarylethenes did not lead to strong electronic interactions that influence the absorption spectra.20The fluorescence spectra of BTFc–BTHo and BTFo–BTHc were similar to those of BTFO4 and BTHTO, which confirmed the assignment of isomer states. Interestingly, the BTFc–BTHc isomer showed strong emission at 605 nm similar to the fluorescence of BTHTOc upon excitation with either 400 or 542 nm light. This result indicates that fluorescence of the BTFc moiety of the heterodimer is quenched by the BTHc moiety due to energy transfer between the two diarylethenes.
Recently, we found that the ring closed form of BTHTO isomerized to its ring open form upon addition of a catalytic amount of Cu(ClO4)2 at room temperature in the dark.35 Although the photochemical ring closure reaction of BTHTO did not occur in the presence of Cu2+ in solution, the activity was recovered when EDTA was added to the solution to bind Cu2+. The photochemical and electrochromic ring-close/-open cycle with Cu2+ and EDTA was repeated five times without a significant decrease in the absorption maximum of BTHTOc.
As shown in Scheme 3, three photoisomeric structures of the heterodimer could be obtained by combinations of the dual chromism of the heterodimer. Utilization of 312 nm light together with 1 equivalent of Cu2+ converted BTFo–BTHo to BTFc–BTHo, where the absorption band of the BTFc moiety was intact in the presence of Cu2+, which is the only isomer that gives a strong emission at 492 nm. The BTFc–BTHc state was obtained from BTFc–BTHo by irradiation at 312 nm followed by recovery of the photoswitching behavior of the BTH moiety by quantitative titration of Cu2+ with EDTA. Moreover, the BTFo–BTHo state could be reset from any other state by exposure to visible light. The solutions of all three photoisomeric structures produced the same absorbance and fluorescence spectra as those induced only by light (Fig. 4b and c).
 | ||
| Scheme 3 Photochemical and electrochemical interconversions among the three photoisomeric structures. | ||
Construction of logic gates, keypad lock, and 1:2 demultiplexer
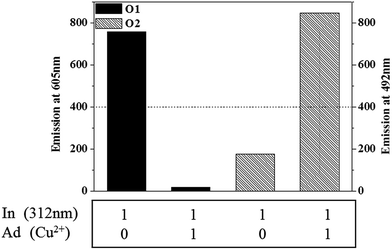
By taking advantage of the multiple optical states of BTF–BTH induced by light, we demonstrated the construction of multifunctional molecular logic gates such as the OR gate (Fig. S1†) and keypad lock. For the keypad lock, two inputs of 312 (U) and 540 nm light (V) were employed to give eight possible two-digit sequence-sensitive codes, as shown in Fig. 5. The output is the fluorescence of BTFc–BTHc at 492 nm upon excitation with 400 nm light. 540 nm light alone does not affect the sample, whereas 312 nm light converts BTFo–BTHo to the BTFc–BTHc form. Thus, none of the first six sets of input orders shown in Fig. 5 result in an output. Likewise, irradiation with 540 nm light followed by 312 nm light generates the BTFc–BTHc form. However, sequential irradiation of BTFo–BTHo with 312 and 540 nm light produces the highly fluorescent BTFc–BTHo state because BTFc–BTHc generated by the first input is isomerized to BTFc–BTHo upon irradiation with 540 nm light. A strong fluorescence at 492 nm can be considered the lock-open signal.BTF–BTH can function as INHIBIT and AND (Fig. S2†) gates and a 1:2 demultiplexer (Scheme 3) under input combinations of 312 nm light and Cu2+. The 1:2 demultiplexer functions as a switch that connects the input signal (In) to two different output lines (O1 and O2) with the help of an address (Ad).43,44 For the purpose of the 1:2 demultiplexer application of BTF–BTH, the input of 312 nm light and a selector of Cu2+ with two output fluorescence signals at 605 nm (O1) and 492 nm (O2) can be employed. When In is applied to the compound without Cu2+ (Ad), strong fluorescence at 605 nm representing the O1-ON state (BTFc–BTHc) will appear. On the other hand, application of both In and Ad causes a fluorescence at 492 nm without fluorescence at 605 nm resulting from the O2-ON/O1-OFF state (BTFc–BTFo). As shown in Fig. 6, when Ad is set to off, O1 reports the state of In, whereas when Ad is switched on, O2 reports the state of In. Thus, a system that can operate as a 1:2 digital demultiplexer function has been established (Table 1).
| In (312 nm) | Ad (Cu2+) | O1 (Em 605 nm) | O2 (Em 492 nm) |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 1 | 0 | 1 | 0 |
| 0 | 1 | 0 | 0 |
| 1 | 1 | 0 | 1 |
Conclusion
We designed and synthesized a multifunctional heterodimer molecule, BTF–BTH, that can exhibit both photochromic and electrochromic properties. The four different isomeric states of the molecule such as BTFo–BTHo, BTFo–BTHc, BTFc–BTHo and BTFc–BTHc were readily established by the irradiation with light of a certain wavelength and Cu2+ ion, and characterized based on the absorption and fluorescence spectra. Various molecular logic gates (OR, keypad lock, INHIBIT, AND and 1:2 demultiplexer) using BTF–BTH were successfully demonstrated.Acknowledgements
This work was supported by a grant from National Research Foundation of Korea (NRF-2014R1A1A2057950).References
- J. Andreasson and U. Pischel, Chem. Soc. Rev., 2010, 39, 174–188 RSC.
- J. Andreasson and U. Pischel, Chem. Soc. Rev., 2015, 44, 1053–1069 RSC.
- T. A. Darwish, R. A. Evans and T. L. Hanley, Dyes Pigm., 2012, 92, 817–824 CrossRef CAS.
- P. Remon, M. Hammarson, S. M. Li, A. Kahnt, U. Pischel and J. Andreasson, Chem.–Eur. J., 2011, 17, 6492–6500 CrossRef CAS PubMed.
- P. Remon, M. Balter, S. M. Li, J. Andreasson and U. Pischel, J. Am. Chem. Soc., 2011, 133, 20742–20745 CrossRef CAS PubMed.
- C. Stoffelen, J. Voskuhl, P. Jonkheijm and J. Huskens, Angew. Chem., Int. Ed., 2014, 53, 3400–3404 CrossRef CAS PubMed.
- R. Afrasiabi and H. B. Kraatz, Chem.–Eur. J., 2015, 21, 7695–7700 CrossRef CAS PubMed.
- A. J. Myles, T. J. Wigglesworth and N. R. Branda, Adv. Mater., 2003, 15, 745–748 CrossRef CAS.
- M. Irie, Chem. Rev., 2000, 100, 1683 CrossRef CAS PubMed.
- M. Irie, T. Fulcaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- S. Kobatake and M. Irie, Tetrahedron, 2003, 59, 8359–8364 CrossRef CAS.
- H. Choi, I. Jung, K. H. Song, K. Song, D. S. Shin, S. O. Kang and J. Ko, Tetrahedron, 2006, 62, 9059–9065 CrossRef CAS.
- S. Kobatake, S. Kuma and M. Irie, J. Phys. Org. Chem., 2007, 20, 960–967 CrossRef CAS.
- A. Perrier, F. Maurel and D. Jacquemin, Acc. Chem. Res., 2012, 45, 1173–1182 CrossRef CAS PubMed.
- C. C. Zhang, S. Z. Pu, Z. Y. Sun, C. B. Fan and G. Liu, J. Phys. Chem. B, 2015, 119, 4673–4682 CrossRef CAS PubMed.
- S. Z. Pu, Z. P. Tong, G. Liu and R. J. Wang, J. Mater. Chem. C, 2013, 1, 4726–4739 RSC.
- S. Z. Pu, D. H. Jiang, W. J. Liu, G. Liu and S. Q. Cui, J. Mater. Chem., 2012, 22, 3517–3526 RSC.
- Q. Zou, X. Li, J. J. Zhang, J. Zhou, B. B. Sun and H. Tian, Chem. Commun., 2012, 48, 2095–2097 RSC.
- S. J. Chen, Y. H. Yang, Y. Wu, H. Tian and W. H. Zhu, J. Mater. Chem., 2012, 22, 5486–5494 RSC.
- J. Andreasson, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, J. Am. Chem. Soc., 2008, 130, 11122–11128 CrossRef CAS PubMed.
- J. Andreasson, U. Pischel, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, J. Am. Chem. Soc., 2011, 133, 11641–11648 CrossRef CAS PubMed.
- M. Balter, S. M. Li, J. R. Nilsson, J. Andreasson and U. Pischel, J. Am. Chem. Soc., 2013, 135, 10230–10233 CrossRef PubMed.
- X. L. Meng, W. H. Zhu, Q. Zhang, Y. L. Feng, W. J. Tan and H. Tian, J. Phys. Chem. B, 2008, 112, 15636–15645 CrossRef CAS PubMed.
- Y. Moriyama, K. Matsuda, N. Tanifuji, S. Irie and M. Irie, Org. Lett., 2005, 7, 3315–3318 CrossRef CAS PubMed.
- N. Xie and Y. Chen, New J. Chem., 2006, 30, 1595–1598 RSC.
- T. Nakashima, Y. Kajiki, S. Fukumoto, M. Taguchi, S. Nagao, S. Hirota and T. Kawai, J. Am. Chem. Soc., 2012, 134, 19877–19883 CrossRef CAS PubMed.
- A. Staykov, J. Areephong, W. R. Browne, B. Feringa and K. Yoshizawa, ACS Nano, 2011, 5, 1165–1178 CrossRef CAS PubMed.
- S. Lee, Y. You, K. Ohkubo, S. Fukuzumi and W. Nam, Org. Lett., 2012, 14, 2238–2241 CrossRef CAS PubMed.
- H. Logtenberg and W. R. Browne, Org. Biomol. Chem., 2013, 11, 233–243 CAS.
- Y. C. Jeong, J. P. Han, Y. Kim, E. Kim, S. I. Yang and K. H. Ahn, Tetrahedron, 2007, 63, 3173–3182 CrossRef CAS.
- Y. C. Jeong, S. I. Yang, E. Kim and K. H. Ahn, Tetrahedron, 2006, 62, 5855–5861 CrossRef CAS.
- Y. C. Jeong, D. G. Park, E. Kim, K. H. Ahn and S. I. Yang, Chem. Commun., 2006, 1881–1883 RSC.
- S. C. Pang, H. Hyun, S. Lee, D. Jang, M. J. Lee, S. H. Kang and K. H. Ahn, Chem. Commun., 2012, 48, 3745–3747 RSC.
- S. Pang, D. Jang, W. S. Lee, H. M. Kang, S. J. Hong, S. K. Hwang and K. H. Ahn, Photochem. Photobiol. Sci., 2015, 14, 765–774 CAS.
- Q. Ai, S. Pang and K.-H. Ahn, Chem.–Eur. J., 2016, 22, 656–662 CrossRef CAS PubMed.
- O. Zavalov, V. Bocharova, V. Privman and E. Katz, J. Phys. Chem. B, 2012, 116, 9683–9689 CrossRef CAS PubMed.
- M. Kumar, A. Dhir and V. Bhalla, Org. Lett., 2009, 11, 2567–2570 CrossRef CAS PubMed.
- C. P. Carvalho, Z. Dominguez, J. P. Da Silva and U. Pischel, Chem. Commun., 2015, 51, 2698–2701 RSC.
- S. J. Chen, Z. Q. Guo, S. Q. Zhu, W. E. Shi and W. H. Zhu, ACS Appl. Mater. Interfaces, 2013, 5, 5623–5629 CAS.
- A. Prokup, J. Hemphill and A. Deiters, J. Am. Chem. Soc., 2012, 134, 3810–3815 CrossRef CAS PubMed.
- R. Orbach, F. Remacle, R. D. Levine and I. Willner, Chem. Sci., 2014, 5, 1074–1081 RSC.
- H. W. Shin, Y. R. Kim and E. Kim, Macromol. Res., 2005, 13, 321–326 CrossRef CAS.
- S. Erbas-Cakmak and E. U. Akkaya, Angew. Chem., Int. Ed., 2013, 52, 11364–11368 CrossRef CAS PubMed.
- J. Andreasson, S. D. Straight, S. Bandyopadhyay, R. H. Mitchell, T. A. Moore, A. L. Moore and D. Gust, J. Phys. Chem. C, 2007, 111, 14274–14278 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06301b |
| This journal is © The Royal Society of Chemistry 2016 |