Fabrication, characterization, bioactivity, and biocompatibility of novel mesoporous calcium silicate/polyetheretherketone composites
G. F. Hua,
R. F. Quanb,
Y. M. Chena,
D. W. Bi*a,
X. S. Jiangc,
X. F. Lic and
J. Y. Li*c
aThe First People's Hospital of Xiaoshang, Hangzhou 311200, China. E-mail: 13758191112@163.com
bXiaoshang Hospital of Traditional Chinese Medicine, Hangzhou 311200, China
cOrthopedics Department, Huzhou Central Hospital, 198 Hongqi Road, Huzhou 313000, China. E-mail: liywn1977@126.com
First published on 8th June 2016
Abstract
The purpose of this study was to develop polyetheretherketone (PEEK)/mesoporous calcium silicate (m-CS) composites (m-CS content: 12 wt% and 24 wt%, abbreviated as CPC12 and CPC24, respectively) to investigate the potential application of CPC for bone tissue regeneration. The compositions and surface morphologies of PEEK, CPC12, and CPC24 were characterized with wide angle X-ray diffraction (WAXRD) and scanning electronic microscopy (SEM), respectively. The water contact angle test demonstrated that the addition of m-CS improved the hydrophilicity of PEEK. The mechanical properties including elastic modulus, compressive strength, and bending strength were enhanced with the addition of m-CS, whereas the tensile strength slightly decreased. The composites, particularly, CPC24, prominently enhanced in vitro bioactivity of PEEK, which was demonstrated by the formation of apatite layer. The in vitro cell culture further suggested that the cell attachment on the composites, especially, CPC24, was significantly higher than PEEK. Encouragingly, the proliferation and ALP activity of the MC3T3-E1 cells were also improved on CPC24 prominently in comparison with the PEEK. Our study indicated that m-CS/PEEK composites, especially, CPC24, had suitable hydrophilicity, bioactivity, and great biocompatibility. Hence, CPC24 would be a great candidate composite with desirable physical and biological properties for bone tissue regeneration.
Introduction
By the late 1990s, PEEK had emerged as the leading high-performance thermoplastic candidate for replacing metal implants, especially in orthopedics and trauma due to its good resistance to in vivo degradation, including damage caused by lipid exposure.1–6 As a result, in April 1998, PEEK was provided commercially as an implant biomaterial.7 However, in terms of biocompatibility and osseointegration, PEEK is often considered as a bioinert material, because PEEK does not release ions, produce byproducts, and corrode or degrade.8–11 Besides, hydrophobic surface, with low surface free energy, of PEEK limits the cellular adhesion.12 Thus, the further application of PEEK in biomedical field encounter bottlenecks, which needs breakthrough in improving biodegradability and bioactivity to integrate with in vivo bone modelling.13Recently, the emphasis on the development of biomaterials has shifted from single material to composites because single material is often accompanied with both merits and demerits simultaneously, hard to fully meet all requirements for application.13 In contrast, by controlling the volume fraction and distribution of phases in the composite, the properties of composites can be tailored to meet mechanical and physiological requirements.14,15
Over the past two decades, calcium silicate-based bioceramics have been introduced as potential bioactive materials for bone tissue regeneration due to the bioactivity and degradability.16 More recently, mesoporous materials, having several attractive structural characteristics, such as high specific surface area, large pore volume, and controllable porosity at meso-scale, have been applied to the fabrication of composite biomaterials, aiming to improve the drawbacks of pure materials while maintain the advantages.17 For example, bioactive mesoporous diopside and poly(L-lactide) composite scaffolds were fabricated by a solution-casting and particulate-leaching method.18 The degradation kinetics, and in vitro and in vivo bioactivity were significantly improved by addition of mesoporous diopside into poly(L-lactide). Similarly, the incorporation of mesoporous wollastonite into polycaprolactone scaffolds also improved the biodegradability, in vitro bioactivity, and in vitro bio-functions evaluated by MG63 cells.19 Consequently, the addition of bioactive mesoporous materials to bioinert materials sheds a shining light on opening a gate to advance the development of bioinert materials for bone tissue engineering.
In our present study, we aim to experimentally and tentatively fabricate bioactive m-CS reinforced PEEK composite, which has not been reported yet, to investigate the potential application of this m-CS/PEEK composite in bone tissue repair. m-CS, as a hydrophilic component, is expected to improve the hydrophilicity of PEEK and modulate the cell functions. Previously, it is demonstrated that surface with a water contact angle of ∼50° can favour cell adhesion and proliferation in comparison with more hydrophilic or more hydrophobic surfaces. Hence, the addition of m-CS into PEEK might achieve a proper hydrophilicity for cell adhesion. Second, the bioactive m-CS might improve the bioactivity of PEEK as well. Last but not least, the addition of m-CS might also change the mechanical properties of PEEK, which influences the cellular events. Thus, the effects of m-CS contents in PEEK on mechanical properties, in vitro bioactivity, and in vitro biocompatibility, will be examined. We believe that our fundamental exploration in this specific direction, improving bioinert material with mesoporous bioactive material, will provide useful data for further application in potential clinical use.
Experimental section
Preparation and characterization of m-CS
The m-CS was synthesized by a modified template-induced and self-assembling method using nonionic block copolymer P123 (EO20PO70EO20, BASF) as the structure directing agent and tetraethylorthosilicate (TEOS, Sigma-Aldrich) as the silica precursor. Briefly, 4.0 g of surfactant P123 was dissolved in 150 mL of 2 M HCl and 50 mL of distilled water while stirring at 37 °C in water bath until the solution became clear. Then, 8.50 g of TEOS and 9.64 g of Ca(NO3)2·4H2O were added to the solution. The mixture was stirred at 37 °C for 24 h, and the resulting precipitate was dried at 80 °C for 12 h in air without any filtering and washing. Afterwards, the m-CS was calcined at 600 °C for 6 h to remove the surfactant template and obtain the final m-CS products, followed by grounding into fine powders. The mesoporous structure of m-CS was confirmed by small-angle X-ray diffraction (SAXRD, Rigaku D/max 2550VB/PC, Japan) and high resolution transmission electron microscopy (HRTEM, JEM-2010, JEOL, Japan). Brunauere–Emmette–Teller (BET) and Barrete–Joynere–Halenda (BJH) analyses were used to determine the structural properties, including surface area, pore size, and pore volume.Preparation and characterization of calcium silicate/PEEK composites (CPC)
The m-CS and PEEK powders (medical grade) were mixed at the desired weight percentages of m-CS (0%, 12%, and 24%) (PEEK, CPC12, and CPC24, respectively) and blended for 4 h to obtain a homogeneous mixture. The mixed powders were die-pressed into disks (ϕ12 mm × 2 mm). The compacts were then sintered at 352 °C for 120 min in an atmospheric furnace. The phase compositions and surface morphologies of samples were characterized by X-ray diffraction (XRD; Rigaku Co., Japan) and scanning electron microscope (SEM, JSM-6360LV, JEOL, Japan) at an accelerating voltage of 15 keV after sputter coating. The surface hydrophilicity of the specimens was assessed by water contact angle measurements performed on a Ramé-Hart (USA) instrument at ambient humidity and temperature. Briefly, 20 μL of water droplet was squeezed onto the sample and the contact angles were recorded after it was stable. The mechanical properties including tensile strength, bending strength, compressive strength, and elastic modulus of the samples were measured using an Instron 5567 material testing machine (Norwood, MA, USA) at a crosshead speed of 0.1 mm min−1 according to ASTM International standard D790 (measurements made in air at room temperature), and average of five readings for each sample was presented.In vitro bioactivity
The in vitro bioactivity of PEEK, CC12, and CC24 was evaluated by immersing the samples in SBF at a concentration of 1 mg mL−1 at 37 °C to monitor the formation of hydroxyapatite (HA) on the surface of samples at 1, 3, 7, and 14 days. The SBF solution was stirred and refreshed every other day. After soaking, the samples were collected from SBF, gently rinsed with deionized water, and dried at room temperature.The surface morphologies and compositions of the samples before and after soaking in the SBF solution were characterized by SEM and energy-dispersive X-ray spectrometer (EDS, INCA Energy, Oxford Instruments, UK). The SBF solutions after soaking for 1, 3, 5, 7, and 14 days were collected for determining the ion concentrations of Ca, P, and Si by inductively coupled plasma atomic emission spectroscopy (ICP-AES; Varian, USA).
Cell attachment and morphology
Samples were sonicated in ethanol and sterilized using the ultra-violet light. MC3T3-E1 cells at a density of 2 × 104 cells per disc were seeded on the samples in 24-well tissue culture plates, followed by culture at 37 °C under 5% CO2 condition for 6, 12, and 24 h. Thereafter, the culture medium was removed at time points and unattached cells were removed by rinse with PBS three times. After the cells on the samples were trypsinized, the adherent cells were counted with a hemacytometer, and the cell attachment efficiency was determined by counting the number of cells remaining in the wells.The cell morphology of the MC3T3-E1 cells was examined by visualizing the filamentous actin of the cytoskeleton using a confocal laser scanning microscopy (CLSM, Leica TCS SP2; Leica Microsystems, Heidelberg, Germany). The cells were allowed to attach for 4 h on the substrates, then 1 mL of fresh culture medium was added to each well, and cells were incubated for 24 h in a humidified atmosphere at 37 °C and 5% CO2. After incubation, the medium was removed and the cells were fixed with 2.5% glutaraldehyde for 15 min and permeabilized with 0.1% Triton X-100 in PBS for another 15 min. After washed with PBS three times, the cells were incubated with FITC-Phalloidin (Abcam, Sigma-Aldrich) for 40 min and then counterstained with DAPI (Molecular Probe, Sigma-Aldrich) to observe the nucleus for 5 min. Finally, the cell morphologies were visualized using the CLSM.
The morphology of MC3T3-E1 cells cultured on the samples for 24 h was also observed by SEM. After cells were fixed as described above, dehydration in a series of graded ethanol (10, 30, 50, 70, 90 and 100% v/v) for 3 min at each concentration was performed. Prior to SEM observation, the specimens were air dried in a desiccator overnight.
Cell proliferation and ALP activity
Cell proliferation at 1, 3, and 7 days was evaluated through culturing cells at a density of 2 × 104 cells per disc with the medium refreshed every 2 days. Cell growth, represented by the optical density (OD), was determined by an MTT assay (MTT Kit, Roche Diagnosis Corp., IN, USA). The absorbance value was measured at 570 nm with a microplate reader (SPECTR Amax 384, Molecular Devices, USA). Three specimens were tested for each incubation period, and each test was performed in triplicate.MC3T3-E1 cells at a density of 5 × 104 per disk were seeded on the samples and cultured as described above. ALP activities at 7, 10, and 14 days were determined according to the manufacturer's instructions (ALP kit 104; Sigma). Briefly, at time points, the cells were washed with PBS. Then, 200 μL of 1% Nonidet P-40 (NP-40) was added to each well and incubated for 1 h to obtain the cell lysate. After centrifugation, 50 μL of the obtained supernatant was transferred to a 96-well plate, mixed with 50 μL of 2 mg mL−1 p-nitrophe-nylphosphate substrate solution composed of 0.1 mol L−1 glycine and 1 mmol L−1 MgCl2, and incubated for 30 min at 37 °C. The reaction was quenched by adding 100 μL of 0.1 N NaOH, and the ALP level was quantified by testing the OD value at a wavelength of 405 nm using a microplate reader. The ALP activity was expressed as the OD value per total protein amount, which was determined using the BCA protein assay kit (Pierce Biotechnology Inc., Rockford, IL, USA). Six specimens were tested for each time point, and each test was performed in triplicate.
Results
The typical SAXRD profile of the m-CS was shown in Fig. 1a, which exhibited a well resolved peak (100) and very weak signals (110, 200), indicating a high degree of hexagonal mesoscopic organization (p6mm). Fig. 1b illustrated the microstructure of m-CS observed by TEM, showing that the m-CS had a well-ordered mesoporous structure with the pore size of ∼5 nm.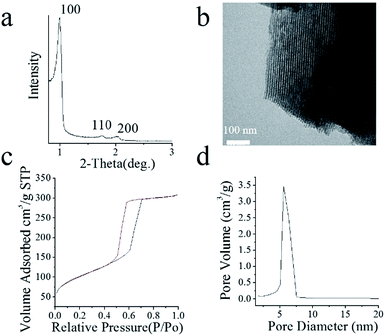 | ||
| Fig. 1 (a) SAXRD spectrum, (b) TEM image of m-CS, (c) N2 adsorption–desorption isotherms, and (d) BJH curve of m-CS. | ||
The mesoporous structure of m-CS was also confirmed by the N2 adsorption–desorption isotherm and Barret–Joyner–Halenda (BJH) adsorption as shown in Fig. 1c and d, respectively. The N2 adsorption–desorption isotherm showed an adsorption at low pressures, an increase in adsorption with increasing pressure, and hysteresis upon desorption, reflecting a type IV isotherm, typical for mesoporous materials with a pore size ranging from 2 to 50 nm according to the Brunauer–Deming–Deming–Teller (BDDT) classification.20 Corresponding to the N2 adsorption–desorption isotherm, the BJH adsorption shows the distribution of pore volume with pore diameter, which enabled us to reckon that the specific surface area and mean pore diameter were 355.6 m2 g−1 and 5.3 nm, respectively. Consistent with TEM images in Fig. 1b, the pore size distribution of m-CS in Fig. 1d indicated m-CS had a uniform mesoporous structure.
Fig. 2 presented the WAXRD patterns of PEEK, CPC12, CPC24, and m-CS. Clearly, the characteristic peaks assigned to PEEK at 18.71°, 20.76°, 22.81°and 28.83° were recognized. With the increase of m-CS content, the peak intensity of PEEK were gradually weakened.
The surface morphologies of PEEK, CPC12, and CPC24 were observed by SEM as demonstrated in Fig. 3. CPC24 showed slightly rougher surface compared with PEEK and CPC12, however, the surfaces of all samples showed similar features with fairly smooth topography.
 | ||
Fig. 3 SEM images of PEEK (a and d), CPC12 (b and e), and CPC24 (c and f) at magnifications of 500× (top row) and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× (bottom row). 000× (bottom row). | ||
The addition of m-CS to the PEEK influenced the hydrophilicity, characterized by water contact angles (CAs), as shown in Fig. 4. The CA decreased from ∼88° on PEEK to ∼80° and ∼72° on CPC12 and CPC24, respectively. The representative photographs of water droplets also demonstrated the consistent results, meaning that the m-CS improved the hydrophilicity efficiently.
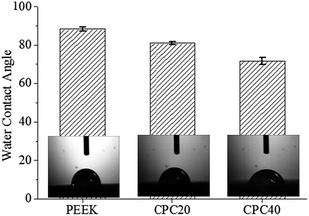 | ||
| Fig. 4 Water contact angles of PEEK, CPC12, and CPC24. Insets: representative water droplets on the corresponding surfaces. | ||
The mechanical properties, including elastic modulus, compressive strength, tensile strength, and bending strength, of PEEK, CPC12, and CPC24 were also investigated as shown in Table 1. With the addition of m-CS, the elastic modulus, compressive strength, and bending strength increased significantly, whereas the tensile strength decreased slightly.
| Samples | Elastic modulus (GPa) | Compressive strength (MPa) | Tensile strength (MPa) | Bending strength (MPa) |
|---|---|---|---|---|
| PEEK | 2.8 ± 0.3 | 109 ± 7.8 | 98 ± 4.5 | 87 ± 6.4 |
| C12 | 4.1 ± 0.4 | 143 ± 8.3 | 97 ± 8.1 | 101 ± 9.8 |
| C24 | 5.5 ± 0.3 | 181 ± 13.6 | 95 ± 9.6 | 123 ± 13.2 |
The prerequisite for biomaterial to bond to living bone is the formation of a bioactive apatite layer on the surface of implant in vivo.18,19 Therefore, in this experiment, the bone-bonding ability of the composites in vitro was tested in SBF to monitor the formation of apatite on the surface as described previously.19 The SEM images in Fig. 5 showed the surface morphology after soaking in SBF for 7 days. With a great difference from PEEK showing no deposits of apatite layer, the surface morphology of CPC12 and CPC24 changed and particularly the CPC24 surface was fully covered with a continuous layer of dense globular deposits, the appearance of apatite. The deposit layer was further examined with EDS detection as shown in Fig. 5d and e, typical spectra obtained from CPC12 and CPC24. Both Ca and P peaks were detected with Ca/P ratios of 1.61 and 1.74 for CPC12 and CPC24, respectively, nearly identical to the theoretical Ca/P ratio of hydroxyapatite, 1.67.
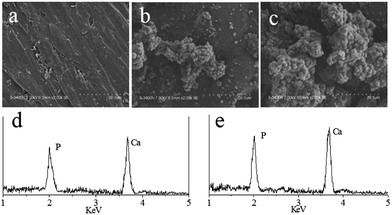 | ||
| Fig. 5 SEM images of PEEK (a), CPC12 (b), and CPC24 (c), and representative EDS spectra of CPC12 (d) and CPC24 (e) after soaking in SBF for 7 days. | ||
Fig. 6 showed the changes in ion concentrations of Ca, P, and Si in SBF solutions with soaking periods for PEEK (a), CPC12 (b), and CPC24 (c), measured by ICP. Both Ca and Si concentrations in SBF increased, while P concentration decreased, indicating the formation of apatite on the surfaces of CPC24 and CPC12 due to the P consumption during apatite formation. However, Ca and P concentrations in SBF solution containing PEEK nearly unchanged.
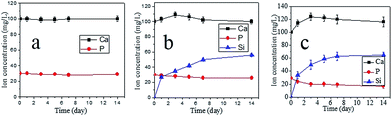 | ||
| Fig. 6 Change of the Ca, P, and Si ion concentrations after soaking PEEK (a), CPC12 (b), CPC24 (c) in SBF solution for different time intervals. | ||
Fig. 7 illustrated the morphology of the MC3T3-E1 cells on PEEK, CPC12, and CPC24 stained by Alexa Fluor 488 phalloidin and DAPI. Evidently, cells on CPC24 displayed limpid F-actin microfilaments after incubation for 24 h, additionally with good spreading on CPC12 and CPC24, which, however, was not observed on PEEK.
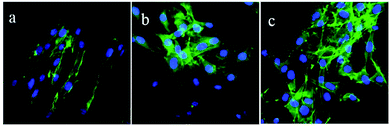 | ||
| Fig. 7 Morphology of MC3T3-E1 cells stained with Alexa Fluor 488 phalloidin (green) and DAPI (blue) on PEEK (a), CPC12 (b), and CPC24 (c) at 24 h. Original magnification: 40×. | ||
The MC3T3-E1 cell morphology at 24 h on PEEK, CPC12, and CPC24 was also observed by SEM as shown in Fig. 8. Consistently, the cells spread and attached well onto the CPC12 and CPC24 surfaces and partly formed a confluent layer, demonstrating that the CPC12 and CPC24 had good biocompatibility and showed no negative effects on cell adhesion and viability.
The normalized cell attachment at 6 h, 12 h, and 24 h were shown in Fig. 9. At 6 h, cell attachment on the CPC24 was significantly higher than that on PEEK and CPC12, but no significant difference was found between PEEK and CPC12. At 12 h and 24 h, the significant difference was found among all samples and the difference was more evident at longer culture time.
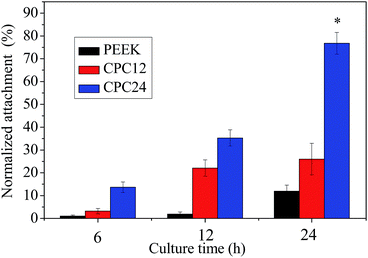 | ||
| Fig. 9 MC3T3-E1 cell attachment on PEEK, CPC12, and CPC24 at 6, 12, and 24 h. *: p < 0.01 relative to others. | ||
The MC3T3-E1 cell proliferation, represented by the optical density (OD) values on PEEK, CPC12, and CPC24 was shown in Fig. 10. The OD value on CPC24 was significantly higher than PEEK and CPC12 on 4 and 7 days and CPC24 was evidently better than CPC12, however, no significant difference was found on 1 day for among PEEK, CPC12, and CPC24. The results showed that the CPC12, and, in particular, CPC24 could promote cell proliferation as compared with PEEK.
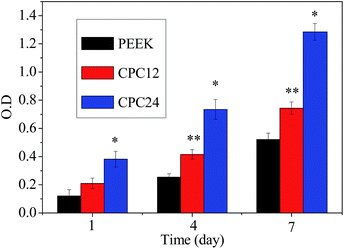 | ||
| Fig. 10 Cell proliferation, represented by OD values determined by MTT assay on PEEK, CPC12, and CPC24 for 1, 4, and 7 days. *: p < 0.01 relative to others. **: p < 0.01 relative to PEEK. | ||
The ALP activity assay is generally used to study biochemical markers for expression of osteoblast activity. The ALP activities of MC3T3-E1 cells on PEEK, CPC12, and CPC24 are shown in Fig. 11. Clearly, the CPC24 and CPC12 showed significantly higher ALP activity than PEEK at 7 days and this difference became larger when culture time increased. However, unlike cell proliferation in Fig. 10, the ALP activity on CPC24, although, was higher than that on CPC12, the difference was not great.
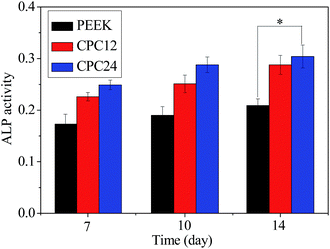 | ||
| Fig. 11 ALP activity of MC3T3-E1 cells cultured on the samples for 7, 10, and 14 days. *: p < 0.05 relative to PEEK. | ||
Discussions
The m-CS has been proved to be biocompatible by previous studies both in vitro and in vivo.21–23 In this study, we have successfully prepared the m-CS material by using a triblock copolymer (P123) as a structure-directing agent. The microstructure and porosity of m-CS were investigated by SAXRD, TEM and the N2 adsorption–desorption technique, which clearly indicated that m-CS possessed a mesoporous structure with a specific surface area of 355.6 m2 g−1 and mean pore diameter of 5.3 nm, consistent with other reports.18,24,25Further, novel bone substitute composites of CPC12 and CPC24 were prepared by mixing m-CS and PEEK. The SEM images acquired from CPC12 and CPC24 showed that m-CS could be integrated into the PEEK component and the higher m-CS content induced coarser surface. In addition, the incorporation of m-CS into PEEK also improved the hydrophilicity greatly. The results suggested that the CA decreased from ∼88° on PEEK to ∼80° and ∼72° on CPC12 and CPC24, respectively. When cells are exposed to culture medium, the interaction between cells and the physical properties of material surface plays a crucial role in guiding early cellular events, including adhesion, migration, proliferation, and differentiation.26–28 The surface energy or the contact angle, as part of the surface properties, influenced the biological performance in vitro and in vivo significantly. It is suggested that the surface with a water contact angle of ∼50° can favour cell adhesion and proliferation in comparison with more hydrophilic or more hydrophobic surfaces.26,27,29 With a contact angle closer to ∼50°, the surface tends to form more biocompatible interface fostering cells. Thus, the hydrophobic PEEK with a contact angle of ∼88° transformed to more hydrophilic composites, in particular, CPC24 with a contact angle of ∼72°, with the addition of m-CS. The improved hydrophilicity on composites, especially CPC24, might contribute to the enhanced cell adhesion and proliferation.
Bioactive materials can bond with living bone through the apatite layers formed on their surfaces once in contact with body fluid after implanted in vivo.30 In this study, the SEM results showed that the amount of apatite formed on CPC24 specimens was much more than that on CPC12 at the same time point, and the PEEK did not show apatite formation for 7 days, indicating that the addition of m-CS efficiently improved the bioactivity of PEEK. To better understand the bioactivity of composites, the ion concentrations in SBF were also studied. The Ca and Si ion concentrations in the SBF solution increased with time in the first 3 days, which should be attributed to the dissolution of Ca and Si ions from the m-CS. It can be concluded that, although the formation of the apatite layer consumed some Ca ions, Ca ion dissolution from the m-CS exceeded its consumption. After 3 days, the degradation rate of Ca ion began to slow down, and the consumption of Ca ion gradually dominated. Therefore, the Ca ion concentration in the SBF solution decreased after 3 days. The P ion concentration in the SBF decreased gradually throughout the soaking period. The decrease in P concentration was attributed to the formation of amorphous calcium phosphate and the subsequent formation of apatite by incorporating OH− ions from the SBF, providing an indirect indication that the apatite precipitation occurred. As suggested in earlier studies, the formation of apatite layer on biomaterials can be considered as an indicator of bioactivity. The addition of m-CS into PEEK, particularly, for CPC24, strongly enhanced the formation of apatite formation, meaning that m-CS, dramatically, improved the bioactivity of PEEK.
To evaluate the in vitro biocompatibility of PEEK, CPC12, and CPC24, the MC3T3-E1 cells were cultured on them to examine the cell attachment, phenotype, proliferation, and differentiation. The cellular responses to a biomaterial, such as attachment, proliferation and differentiation, depend on not only physical properties, such as surface morphology, but also chemical composition of the material. After 24 h, the cells spread well with an intimate contact with the surface of the composites, and partially covered the surface of the composites as demonstrated in Fig. 10. Again, the results showed that the MC3T3-E1 cells could not only proliferate on the composites with culture time, as demonstrated by the MTT assay, suggesting good biocompatibility, but also presented faster growth rate on composites, in particular, CPC24. Furthermore, the ALP activity, an early marker for functionality and differentiation of osteoblasts, exhibited significantly higher levels on composites, especially CPC24 at 7 days, 10 days, and 14 days, and the difference became bigger with incubation time.
The enhanced cell attachment, proliferation, and differentiation can be interpreted in several aspects. First, the chemical composition of biomaterials exerted an impact on guiding cellular events, through release of inorganic ions, such as Ca, P, and Si here, further applying subtle effect on the cell–material interaction.31 Previously, the dissolution products, including Ca and Mg ions, of bioactive glasses and ceramics promoted osteoblast proliferation and differentiation.32 In our present study, similarly, Ca and Si release from the m-CS embedded in composites, in particular CPC24, might contribute to the enhanced cell proliferation on composites, as compared with PEEK. Benefiting from the high specific surface area and pore volume of m-CS, large contact surface area of m-CS might be exposed to culture medium, supplying more ions to support cell functions. Hence, CPC24 with higher m-CS content, accordingly, showed better cell adhesion and proliferation. Note that MC3T3-E1 cell differentiation launched after confluence achieved. As a consequence of proliferation, the cells on CPC24 obtained confluence earlier than others and showed better differentiation than other samples. Second, Ca ion is suggested to exert a crucial influence on ERK1/2 activation, which is part of extracellular signal-regulated kinases (ERKs) in osteoblasts. Earlier, Ca ions were found to enhance ALP activity and mineralization process of MC3T3-E1 cells.33 Moreover, the P ion, a special signal for skeletal cells, also plays a role in regulating cell functions.34 Third, the approximate surface hydrophilicity of CPC24 improved by incorporation of m-CS might enhance the cell adhesion and proliferation, which further stimulated the cell differentiation. Last but not least, the mechanical properties were also modulated by the addition of m-CS into PEEK. It has been demonstrated that the compressive strength of dense ceramic biomaterials matches close to the cortical bone (100–200 MPa), and different polymers to that of cancellous bone (2–20 MPa), however bioceramic/polymer composite scaffolds are typically weaker than bone.35,36 With addition of m-CS into PEEK, the compressive strength was significantly enhanced to ∼181 MPa for CPC24, which was more comparable to the cortical bone. This improved mechanical properties might also contribute to the promoted cellular response on CPC12 and, particularly, CPC24. Hence, the improved hydrophilicity, mechanical properties, and bioactivity strongly push the CPC24 to be a great candidate composite for bone tissue repair.
Conclusions
Novel composites consisting of PEEK and m-CS, CPC12 and CPC24, were fabricated in this study. The sample soaking in SBF solution proved that composites, particularly, CPC24 possessed excellent in vitro bioactivity compared with PEEK, which was confirmed from the formation of apatite layer. The in vitro cell culture indicated that the cell attachment on the composites, especially, CPC24, significantly higher than PEEK. Similarly, the proliferation and ALP activity of the MC3T3-E1 cells were also improved on CPC24 prominently in comparison with the PEEK. Our study indicated that m-CS/PEEK composites, especially, CPC24, had suitable hydrophilicity, mechanical properties, bioactivity and, more importantly, promoted MC3T3-E1 cell attachment, proliferation and differentiation. Definitely, CPC24 would be a great candidate composite with many advanced physical and biological properties for bone tissue regeneration.Acknowledgements
This study was supported by grants from Zhejiang Provincial Natural Science Foundation of China (LY15E020001), Medical Scientific Research Foundation of Zhejiang Province (2014PYA021).Notes and references
- Y. Zhao, H. M. Wong, W. Wang, P. Li, Z. Xu, E. Y. Chong, C. H. Yan, K. W. Yeung and P. K. Chu, Biomaterials, 2013, 34, 9264 CrossRef CAS PubMed.
- S. Yu, K. P. Hariram, R. Kumar, P. Cheang and K. K. Aik, Biomaterials, 2005, 26, 2343 CrossRef CAS PubMed.
- S. M. Kurtz and J. N. Devine, Biomaterials, 2007, 28, 4845 CrossRef CAS PubMed.
- J. D. Lambert, D. H. Kim, R. Zheng and C. S. Yang, J. Pharm. Pharmacol., 2006, 58, 599 CrossRef CAS PubMed.
- R. Zheng, A. C. Dragomir, V. Mishin, J. R. Richardson, D. E. Heck, D. L. Laskin and J. D. Laskin, Toxicol. Appl. Pharmacol., 2014, 279, 43 CrossRef CAS PubMed.
- R. Zheng, D. E. Heck, A. T. Black, A. Gow, D. L. Laskin and J. D. Laskin, Free Radical Biol. Med., 2014, 67, 1 CrossRef CAS PubMed.
- V. Lorber, A. C. Paulus, A. Buschmann, B. Schmitt, T. M. Grupp, V. Jansson and S. Utzschneider, J. Mater. Sci.: Mater. Med., 2014, 25, 141 CrossRef CAS PubMed.
- M. S. Abu Bakar, M. H. W. Cheng, S. M. Tang, S. C. Yu, K. Liao, C. T. Tan, K. A. Khor and P. Cheang, Biomaterials, 2003, 24, 2245 CrossRef CAS PubMed.
- K. B. Sagomonyants, M. L. Jarman-Smith, J. N. Devine, M. S. Aronow and G. A. Gronowicz, Biomaterials, 2008, 29, 1563 CrossRef CAS PubMed.
- R. Zheng, D. E. Heck, V. Mishin, A. T. Black, M. P. Shakarjian, A. N. Kong, D. L. Laskin and J. D. Laskin, Toxicol. Appl. Pharmacol., 2014, 275, 113 CrossRef CAS PubMed.
- R. Zheng, I. Po, V. Mishin, A. T. Black, D. E. Heck, D. L. Laskin, P. J. Sinko, D. R. Gerecke, M. K. Gordon and J. D. Laskin, Toxicol. Appl. Pharmacol., 2013, 272, 345 CrossRef CAS PubMed.
- S. Pavlica, A. Piscioneri, F. Peinemann, M. Keller, J. Milosevic, A. Staeudte, A. Heilmann, M. Schulz-Siegmund, S. Laera, P. Favia, L. De Bartolo and A. Bader, Biomaterials, 2009, 30, 6514 CrossRef CAS PubMed.
- A. H. Poulsson, D. Eglin, S. Zeiter, K. Camenisch, C. Sprecher, Y. Agarwal, D. Nehrbass, J. Wilson and R. G. Richards, Biomaterials, 2014, 35, 3717 CrossRef CAS PubMed.
- P. R. Schmidlin, B. Stawarczyk, M. Wieland, T. Attin, C. H. Hammerle and J. Fischer, Dent. Mater., 2010, 26, 553 CrossRef CAS PubMed.
- A. Iulianelli, I. Gatto, E. Passalacqua, F. Trotta, M. Biasizzo and A. Basile, Int. J. Hydrogen Energy, 2013, 38, 16642 CrossRef CAS.
- S. Spange, A. Graser, A. Huwe, F. Kremer, C. Tintemann and P. Behrens, Chemistry, 2001, 7, 3722 CrossRef CAS PubMed.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS.
- Z. Liu, et al., J. R. Soc., Interface, 2015, 12(111) DOI:10.1098/rsif.2015.0507.
- J. Wei, F. Chen, J.-W. Shin, H. Hong, C. Dai, J. Su and C. Liu, Biomaterials, 2009, 30, 1080 CrossRef CAS PubMed.
- C. A. Fyfe and G. Fu, J. Am. Chem. Soc., 1995, 117, 9709 CrossRef CAS.
- C. Li, L. Gao, F. Chen and C. Liu, J. Mater. Sci., 2015, 50, 7182 CrossRef CAS.
- C. Kang, Y. Sun, M. Wang and X. Cheng, Eur. J. Med.Res., 2016, 2, 7 CrossRef.
- Y. Sun, C. Kang, A. Zhang, F. Liu, J. Hu, X. Zhong and J. Xie, Eur. J. Med.Res., 2016, 2, 7 CrossRef.
- A. P. Waller, M. George, A. Kalyanasundaram, C. Kang, M. Periasamy, K. Hu and V. A. Lacombe, Biochim. Biophys. Acta, 2013, 1832, 121 CrossRef CAS PubMed.
- B. C. Yung, J. Li, M. Zhang, X. Cheng, H. Li, E. M. Yung, C. Kang, L. E. Cosby, Y. Liu, L. Teng and R. J. Lee, Mol. Pharm., 2016, 13, 653 CrossRef CAS PubMed.
- K. Anselme, P. Davidson, A. M. Popa, M. Giazzon, M. Liley and L. Ploux, Acta Biomater., 2010, 6, 3824 CrossRef CAS PubMed.
- C. J. Bettinger, R. Langer and J. T. Borenstein, Angew. Chem., Int. Ed., 2009, 48, 5406 CrossRef CAS PubMed.
- Z. Han, Z. Zhou, X. Shi, J. Wang, X. Wu, D. Sun, Y. Chen, H. Zhu, C. Magi-Galluzzi and Z.-R. Lu, Bioconjugate Chem., 2015, 26, 830 CrossRef CAS PubMed.
- Z. Zhou, et al., Nat. Commun., 2015, 6 DOI:10.1038/ncomms8984.
- J. M. Oliveira, R. N. Correia and M. H. Fernandes, Biomaterials, 2002, 23, 371 CrossRef CAS PubMed.
- A. Ito, H. Kawamura, S. Miyakawa, P. Layrolle, N. Kanzaki, G. Treboux, K. Onuma and S. Tsutsumi, J. Biomed. Mater. Res., 2002, 60, 224 CrossRef CAS PubMed.
- G. Daculsi, E. Goyenvalle, R. Cognet, E. Aguado and E. O. Suokas, Biomaterials, 2011, 32, 3166 CrossRef CAS PubMed.
- H. Sowa, H. Kaji, T. Yamaguchi, T. Sugimoto and K. Chihara, J. Bone Miner. Res., 2002, 17, 1190 CrossRef CAS PubMed.
- X. H. Wu, Z. Y. Wu, J. Qian, Y. G. Yan, J. Wei, H. Li and J. C. Su, RSC Adv., 2015, 5, 36007 RSC.
- M. J. Olszta, X. Cheng, S. S. Jee, R. Kumar, Y.-Y. Kim, M. J. Kaufman, E. P. Douglas and L. B. Gower, Mater. Sci. Eng., R, 2007, 58, 77 CrossRef.
- C. P. Jiang, Y. Y. Chen, M. F. Hsieh and H. M. Lee, Biomed. Microdevices, 2013, 15, 369 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |