Crosslinking catalysis-active center of hemin on the protein scaffold toward peroxidase mimic with powerful catalysis†
Abstract
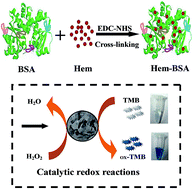
An enzyme mimic synthesis protocol has been proposed by simply cross-linking the redox active center of peroxidase onto a protein scaffold. Colorimetric assays and kinetic studies indicate that the developed peroxidase mimic can present much stronger catalysis and better aqueous stability than native hemin.

 Please wait while we load your content...
Please wait while we load your content...