Supramolecular assembly-mediated lithium ion transport in nanostructured solid electrolytes†
Chih-Chia Cheng*a and
Duu-Jong Leebcd
aGraduate Institute of Applied Science and Technology, National Taiwan University of Science and Technology, Taipei 10607, Taiwan. E-mail: cccheng@mail.ntust.edu.tw
bDepartment of Chemical Engineering, National Taiwan University, Taipei 10617, Taiwan
cDepartment of Chemical Engineering, National Taiwan University of Science and Technology, Taipei 10607, Taiwan
dR&D Center for Membrane Technology, Chung Yuan Christian University, Chungli, Taoyuan 32043, Taiwan
First published on 13th April 2016
Abstract
Multiple hydrogen-bonded supramolecular solid electrolytes forming phase-separated lamellar nanostructures are used as an ion migration path for the effective transport of lithium within the well-ordered lamellar nanochannels, thus providing a new route to fabricate high-performance supramolecular electrolytes with tunable ionic conduction and mechanical properties.
In recent decades, rechargeable lithium-ion batteries have been extensively used in various electronic devices and transportation applications as they offer several advantages, such as easy preparation, low cost, as well as high power and energy density.1–4 Conventional lithium-ion batteries utilize liquid electrolytes that provide excellent electrochemical performance with high ionic conductivity.5 However, liquid electrolytes have a number of drawbacks, including leakage of liquid media, flammable electrolytes, sensitivity to varied operating temperatures, and poor dimensional stability and mechanical properties.6,7 Solid polymer electrolytes (SPEs) represent a desirable approach to solve the above-mentioned problems and have been widely investigated as safe electrolytes for applications in solvent-free lithium batteries due to their features of high safety in high-charge/discharge situations, i.e., overpressure relief and overheating protection.6,8–10 Although many types of synthetic SPEs that allow charge carriers to move quickly between two electrodes have been developed, these SPEs have a characteristic crystalline structure, leading to a low ionic conductivity at room-temperature.11–13 This drawback of low conductivity can be overcome by using low-molecular-weight SPEs to reduce the degree of crystallinity. However, these SPEs tend to exhibit insufficient mechanical properties and dimensional stability due to the highly viscous liquid and wax-like products.14,15 Identification of suitable material systems and the use of appropriate approaches that simultaneously improve both the mechanical and ion conductive properties of SPEs remains a grand challenge that prevents the development of safe, high-performance lithium-ion batteries.
To address the issues described above and improve existing technologies for lithium-ion batteries, supramolecular liquid-crystalline assemblies (SLCA) have been demonstrated to form well-ordered columnar stacks or one-dimensional nanostructures that promote efficient development of supramolecular ion-conductive materials. These materials exhibit enhanced ionic transport, increased electronic conductivity and temperature-induced phase transition of self-assembled aggregates, which can be directly manipulated to obtain a wide variety of energetic nanostructures with diverse dimensions and sizes.16–26 Recently, these materials have been used to power energy-harvesting devices such as fuel cells, lithium-ion batteries and dye-sensitized solar cells.27–29 However, a major drawback of these SLCA-mediated materials is the fact the presence of various nanostructures in self-assembled liquid crystal systems requires a delicate balance to be struck between intermolecular interactions and the polar/non-polar components of the materials, and thus effective control of the self-assembly process towards targeted nanostructures remains an unresolved issue.30,31 Due to the specific obstacles mentioned above, supramolecular polymers have been strongly recommended to be able to control the self-assembly process and stability of well-defined secondary nanostructures with different dimensions.32–35 The incorporation of strong noncovalent interactions within synthetic supramolecular polymers provides a method of creating multi-functional materials from these basic building blocks and results in polymers with the required properties and superior workability.36,37 In addition, manipulation of self-assembled nanostructures within the matrices of polymer architectures could possibly be used as a means of correlating ion channel activation with a subsequent increase in ion transport rates; this approach could potentially enhance the ionic conductivity and performance of lithium-ion batteries and result in improved long-term mechanical stability. Therefore, multifunctional supramolecular SPEs constructed using various noncovalent interactions may have great potential for achieving high-performance electrolytes for a variety of applications in energy conversion and storage devices.
We recently developed a new process to obtain urea-cytosine end-capped polypropylene glycol 800 (UrCy-PPG, ca. 14 repeat units) for large-scale production of high-quality supramolecular polymers.38 A new class of supramolecular polymer containing urea-cytosine (UrCy) moieties shows high association constants (Ka > 106 M−1) and excellent self-assembly behavior in solution and the solid-state. We found that the UrCy-moieties are essential elements that guide and drive UrCy-PPG macromers to spontaneously assemble into a long-range ordered lamellar nanostructure with a periodicity of around 7 nm; this material can be directly used to exfoliate graphite powders into high concentrations of uniform graphene due to the efficient interaction between UrCy-PPG and graphene.39 This supramolecular polymer can also be transformed into a physically crosslinked structure with unique stimuli-responsiveness and self-healing properties.40 Based these findings, we reasonably hypothesized that dissociation of ion salts within self-assembled lamellar UrCy-PPG matrices may significantly affect the migration of lithium ions across a flexible segment of the lamellae and result in both high ionic conductivity and mechanical performance, and represent a promising strategy for the development of highly ordered ion-conducting materials with greatly improved electrochemical characteristics. To date, this is the first report of low-molecular-weight supramolecular SPEs that can effectively establish well-defined two-dimensional ionic channels to manipulate ion transport within lamellar structures at the nanoscale. Thus, this provides new insights into tailoring structural stability and ionic migration behavior to enhance ionic conductivity via supramolecular self-assembly of lithium-ion battery electrolytes. Herein, we demonstrate that the UrCy-PPG macromer can also be employed as an SPE material that possesses free-flowing ion-conductive pathways due to its novel organization nanostructure. After addition of the lithium salt, synchrotron wide-angle X-ray scattering (WAXS) and solid-state nuclear magnetic resonance (NMR) studies showed no apparent change in the lamellar structure of the resulting SPEs across a broad concentration range of additive lithium salts (3 wt% to 13 wt%), which indicates that lithium ions prefer to arrange at and move along lamellar boundaries. More importantly, the generated SPEs exhibited excellent ionic conductivities and tunable mechanical properties, which can be attributed to an orderly phase-separated phase stabilized by the rigid UrCy moieties within the closely-packed supramolecular structure (Scheme 1). Hence, these newly-developed supramolecular SPEs open an entirely new avenue to improve the performance of lithium-ion batteries and satisfy the ever-changing demands of the global electronics industry.
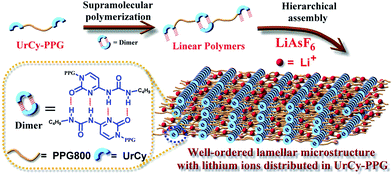 | ||
| Scheme 1 Graphical illustration of the co-assembly process UrCy-PPG/LiAsF4 complexes in the bulk state. | ||
A simple route for the preparation of UrCy-PPG/lithium hexafluoroarsenate (LiAsF4) electrolytes is shown in Scheme 1, and involves a solution blending method that promotes dissociation of a lithium salt via its complex with UrCy-PPG in tetrahydrofuran (THF) solvent. Due to the strong ionic bonds between LiAsF4 molecules, increasing the blending time was found to be an important step that simultaneously promotes ionic dissociation and hierarchical assembly between the UrCy-PPG and LiAsF4 materials. After blending at 25 °C for 1 day, a composite film could be readily prepared by casting the sample solution onto a clean glass substrate, followed by drying under ambient conditions and thermal annealing at 100 °C. In terms of appearance, the UrCy-PPG and LiAsF4 materials behaved like solid powders and the LiAsF4/UrCy-PPG blend produced a white-colored, opaque, elastic, thin-film (Scheme S1†). This implied that a complementary structure exists between LiAsF4 and UrCy-PPG that greatly influences the macroscale phenomenon of supramolecular SPEs, prompting us to further investigate how this specific interaction affects the self-assembly behavior. Therefore solid-state 7Li NMR experiments were performed to analyze the microstructural relationship of the UrCy-PPG/LiAsF4 composites, as shown in Fig. 1. Interestingly, the observed 7Li NMR spectra of all composites displayed a clear peak at about −4.71 ppm (Fig. 1a), which is consistent with the formation of a selective association between the ether oxygen segments of polypropylene glycol (PPG) and lithium ions.41 In controlled experiments, the 7Li NMR spectra of the poly(4-vinylpyridine), (P4VP)/LiAsF4, and poly(4-vinylbenzyl uracil),42,43, (PVBU)/LiAsF4, blends showed that these peaks shifted substantially downfield to 0.44 and −0.89 ppm, indicating that most lithium ions interacted with the pyridine groups of P4VP and imide groups of PVBU, respectively (Scheme S2†).20,44,45 These results demonstrate that the lithium ions added into the UrCy-PPG only interacted with the PPG segments without affecting the self-complementary interaction of the UrCy motifs, resulting in highly ordered micro-phase separated structure.14–16
 | ||
| Fig. 1 (a) Solid-state 7Li NMR spectra and (b) WAXS data for LiAsF4, UrCy-PPG and UrCy-PPG/LiAsF4 hybrid composite at 25 °C. | ||
Further investigation of the microstructures of these SPEs was carried out using WAXS. Pristine UrCy-PPG exhibited a long-range ordered lamellar structure (q = 1 to 3) and the well-defined crystalline domains of UrCy moieties were observed in regular, close-packed arrays, as shown in the WAXS patterns in Fig. 1b. Surprisingly, the 32/1 and 16/1 UrCy-PPG/LiAsF4 composites showed long-period stacking ordered structures with characteristic crystal peaks, indicating the presence of orderly-packed UrCy segments and lamellar microstructures.38 On the other hand, incorporating a low ratio of LiAsF4 into the UrCy-PPG matrix did not affect the self-assembly ability of UrCy-PPG due to the high dimerization constant of the UrCy unit.46 However, as the LiAsF4 content increased to a 4/1 ratio, the periodic peaks were not observed in the WAXS patterns, implying that excess lithium ions tend to destroy the hydrogen-bonded structure of the UrCy-PPG macromer, resulting in a homogeneous phase in composite matrices.45 Thus, the optimal blending ratio of UrCy-PPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiAsF4 for effective production of nanostructured lithium ion-conductive SPEs and optimization of lithium-ion content is 16
LiAsF4 for effective production of nanostructured lithium ion-conductive SPEs and optimization of lithium-ion content is 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Based on the 7Li NMR and WAXS analyses, it also appears that the hydrogen-bonded UrCy domain and lithium ion–PPG complex play key roles as two separate phases that dramatically increase the ability to form long-range-ordered hybrid structures (Scheme 1). These observations led us to further explore the thermal and mechanical properties of UrCy-PPG/LiAsF4 composites in more detail using differential scanning calorimetry (DSC) and universal testing machine measurements.
1. Based on the 7Li NMR and WAXS analyses, it also appears that the hydrogen-bonded UrCy domain and lithium ion–PPG complex play key roles as two separate phases that dramatically increase the ability to form long-range-ordered hybrid structures (Scheme 1). These observations led us to further explore the thermal and mechanical properties of UrCy-PPG/LiAsF4 composites in more detail using differential scanning calorimetry (DSC) and universal testing machine measurements.
Fig. 2a shows the DSC thermograms for various UrCy-PPG/LiAsF4 composites. UrCy-PPG exhibited a distinct glass transition temperature (Tg) at around −47.8 °C and a melting temperature (Tm) of 100.2 °C, indicating its semicrystalline nature. For all formed composites, incorporation of LiAsF4 into UrCy-PPG led to significant increases in Tg but did not affect the Tm of UrCy-PPG, indicating that lithium ions selectively interact with oxygen atoms in the PPG segments of the UrCy-PPG matrix. In addition, the Tgs for the UrCy-PPG/LiAsF4 composites increased gradually from −1.2 to 76.8 °C as the LiAsF4 content increased from 3.1 wt% to 25 wt%. This suggests that the PPG-lithium ion complexes form physically cross-linked networks,47,48 which limit polymer chain mobility and thus substantially increase the Tg of the composite. In the case of the 4/1 UrCy-PPG/LiAsF4 composites, the Tm disappeared completely as the LiAsF4 content exceeded a critical value (25 wt%), resulting in a homogeneous amorphous state. This result was evidenced by the fact that the quadruple hydrogen-bonding UrCy-moieties dissociated when excess lithium ions were added, suggesting a change in the hydrogen-bonding ability of UrCy-PPG. The DSC data were also consistent with the findings of the WAXS experiments (Fig. 1b). In order to further understand the structural-mechanical relationship of the UrCy-PPG/LiAsF4 composites, tensile-strength tests were carried out at 25 °C and 40% relative humidity. As shown in Fig. 2b, the ultimate tensile strength of the 32/1 UrCy-PPG/LiAsF4 film was 3.13 ± 0.12 MPa with an elongation at break of 10.9 ± 1.4%, indicating that a low LiAsF4 content tends to increase the flexibility of the thin film due to the presence of a phase-separated structure in the matrix. Further increasing the LiAsF4 content to an 8/1 ratio resulted in a substantial increase in tensile strength, surpassing 16 MPa, whereas the elongation at break decreased significantly to 1.1 ± 0.4%. This suggests that plastic property dominates elastic deformation in the presence of a large amount of LiAsF4 within the composite matrix. In other words, these composites readily assembled into different extents of physically-hierarchical structures; appropriate manipulation of supramolecular interactions at various UrCy-PPG/LiAsF4 blend ratios revealed that the mechanical properties and dimensional stability of SPE films can be readily designed and tailored to meet different requirements. Thus, the simplicity of tuning the thermal and mechanical characteristics of the UrCy-PPG/LiAsF4 films support their suitability for potential application in high-safety lithium-ion batteries, particularly for devices that function at elevated temperatures.
To further evaluate the ion-transporting capacity of the self-assembled nanostructures, the ionic conductivities of UrCy-PPG and UrCy-PPG/LiAsF4 films were measured over a range of temperatures from 30 to 80 °C using an impedance analyzer.20 The temperature-dependent ionic conductivity curves are shown in Fig. 3. Pristine UrCy-PPG film exhibited a low ionic conductivity (10−13 S cm−1) over a wide range of temperatures, due to its natural electrical insulator characteristic. When various amounts of LiAsF4 were added to UrCy-PPG, each UrCy-PPG/LiAsF4 film showed a significant increase in ionic conductivity at 30 °C. This observation can be attributed to the presence of mobile species in the system leading to enhanced ionic mobility and conductivity. Notably, the 32/1 and 16/1 UrCy-PPG/LiAsF4 films exhibited high conductivities of 10−6 to 10−7 S cm−1 at 30 °C, substantially higher than that of the 8/1 and 4/1 UrCy-PPG/LiAsF4 films (2.5 × 10−9 and 2.7 × 10−12 S cm−1 for the 8/1 and 4/1 films, respectively) under the same conditions, which implies that lamellar nanostructured channels offer rapid lithium ion diffusion pathways and act as additional lithium-insertion sites. However, when excess lithium ions were added to UrCy-PPG, ionic conductivity significantly decreased as a result of the constrained mobility of the rigid composite matrix and destruction of the UrCy-PPG nanostructure. Upon heating from 30 to 80 °C, all the composites showed a gradual increase in ionic conductivity with increasing temperature. Surprisingly, the 32/1 and 16/1 UrCy-PPG/LiAsF4 films displayed markedly enhanced ionic conductivity (up to ∼10−4 S cm−1) at 80 °C. Although the presence of UrCy moieties strongly promotes aggregation and micro-phase separation in the solid state PPG-rich phase, the 32/1 and 16/1 films were better able to promote chain mobility during the heating process, resulting in higher ionic conductivity compared to their original states at 30 °C. In addition, these results also imply that UrCy-PPG/LiAsF4 films may improve the safety and thermal stability of lithium-ion batteries under these high-temperature operating conditions, as the hydrogen-bonded segments of the UrCy moieties dissociate at around 100 °C (Fig. 2a). To further understand the enhanced conductivity of these films, especially at high temperatures and during the heating process, the activation energy (Ea) of ionic conduction was estimated from the Arrhenius plots.49 As shown in Fig. 3, Ea increased gradually as the LiAsF4 content increased. For example, the Ea value for the 32/1 UrCy-PPG/LiAsF4 film was 41.2 kJ mol−1 compared to 49.8 kJ mol−1 for the 16/1 UrCy-PPG/LiAsF4 film. In the case of the 8/1 and 4/1 UrCy-PPG/LiAsF4 films with a high lithium-ion content, the Ea values were 62.4 kJ mol−1 and 78.9 kJ mol−1, respectively. These increases in Ea induced by adding excess LiAsF4 into the blend were attributed to the phase transition from an orderly phase-separated structure to a homogeneously cross-linked structure, which thus decreases ionic conductivity due to the corresponding reductions in free volume and the respective ionic and segmental mobilities. Therefore, the presence of phase-separated nanostructures not only provides flexible ionic conduction channels, but also significantly enhanced ionic conductivity and improved mechanical properties and dimensional stability, which indicates UrCy-PPG/LiAsF4 films may represent a potential SPE system for the development of safer, more effective, reliable lithium-ion batteries.
 | ||
| Fig. 3 Temperature-dependence of the ionic conductivities of the UrCy-PPG/LiAsF4 films at 30% relative humidity. | ||
Conclusions
To conclude, we have successfully developed a new bottom-up assembly strategy that generates a supramolecular nanostructure to attain the desired SPEs; hydrogen-bonded supramolecular polymers provide well-controlled nanostructured media for lithium ion transport and exhibit a phase-separated lamellar morphology at various lithium ion ratios. These newly-discovered SPEs can spontaneously self-assemble into highly-ordered nanostructures with significantly enhanced thermal stability and good film-forming properties. Due to the ease of fine-tuning the lithium content of these composites and the presence of a sufficient self-supporting ability, these films can be easily tailored to meet specific mechanical performances, which is a highly desirable feature that is extremely rare among SPE materials. In addition, the produced SPEs films exhibit high ionic conductivity (10−4 to 10−6 S cm−1), low activation energy and promising ionic conductivities at elevated temperatures, suggesting the potential of supramolecular SPEs for application as a suitable candidate material for developing high-performance and safer lithium-ion batteries. Overall, this study shows that UrCy-PPG-based SPEs can be exploited to make a significant breakthrough in achieving low cost, mass production of novel nanostructured electrolytes,38 and provide mechanical integrity and well-defined ion-conducting paths for rapid ion transport that can be applied in high-performance energy devices. Our efforts to incorporate lithium salts into various UrCy-functionalized polymer derivatives and exploration of the factors influencing the performance of lithium-ion batteries have been making steady progress and the results will be reported in due course.Acknowledgements
We thank National Synchrotron Radiation Research Center (NSRRC, Taiwan) for the support in the WAXS measurements. This study was supported financially by “Aim for the Top University Plan” of the National Taiwan University of Science and Technology, and the Ministry of Science and Technology, Taiwan (contract no. MOST 103-2218-E-011-012 and MOST 104-2221-E-011-153).Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- B. Scrosati, Chem. Rec., 2005, 5, 286 CrossRef CAS PubMed.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 CAS.
- J. B. Goodenough and K.-S. Park, J. Am. Chem. Soc., 2013, 135, 1167 CrossRef CAS PubMed.
- B. Scrosati and J. Garche, J. Power Sources, 2010, 195, 2419 CrossRef CAS.
- W. H. Meyer, Adv. Mater., 1998, 10, 439 CrossRef CAS PubMed.
- R. C. Agrawal and G. P. Pandey, J. Phys. D: Appl. Phys., 2008, 41, 223001 CrossRef.
- A. M. Stephan and K. S. Nahm, Polymer, 2006, 47, 5952 CrossRef CAS.
- Z. S. Shui, J. Power Sources, 2007, 164, 351 CrossRef.
- J. Zhang, L. Yue, P. Hu, Z. Liu, B. Qin, B. Zhang, Q. Wang, G. Ding, C. Zhang, X. Zhou, J. Yao, G. Cui and L. Chen, Sci. Rep., 2014, 4, 6272 CrossRef CAS PubMed.
- D. E. Fenton, J. M. Parker and P. V. Wright, Polymer, 1973, 14, 589 CrossRef CAS.
- I. E. Kelly, J. R. Owen and B. C. H. Steele, J. Power Sources, 1985, 14, 13 CrossRef CAS.
- F. M. Gray, J. R. MacCallum and C. A. Vincent, Solid State Ionics, 1986, 18–19, 282 CrossRef CAS.
- N. A. A. Rossi, Z. Zhang, Y. Schneider, K. Morcom, L. J. Lyons, Q. Wang, K. Amine and R. West, Chem. Mater., 2006, 18, 1289 CrossRef CAS.
- L. Zhang, Z. Zhang, S. Harring, M. Straughan, R. Butorac, Z. Chen, L. Lyons, K. Amine and R. West, J. Mater. Chem., 2008, 18, 3713 RSC.
- V. Percec, G. Johansson, J. Heck, G. Ungar and S. V. Batty, J. Chem. Soc., Perkin Trans. 1, 1993, 1, 1411 RSC.
- M. Yoshio, T. Mukai, K. Kanie, M. Yoshizawa, H. Ohno and T. Kato, Adv. Mater., 2002, 14, 351 CrossRef CAS.
- M. Yoshio, T. Mukai, H. Ohno and T. Kato, J. Am. Chem. Soc., 2004, 126, 994 CrossRef CAS PubMed.
- P. H. J. Kouwer and T. M. Swager, J. Am. Chem. Soc., 2007, 129, 14042 CrossRef CAS PubMed.
- Y. C. Yen, C. C. Cheng, S. W. Kuo and F. C. Chang, Macromolecules, 2010, 43, 2634 CrossRef CAS.
- E. S. Hatakeyama, B. R. Wiesenauer, C. J. Gabriel, R. D. Noble and D. L. Gin, Chem. Mater., 2010, 22, 4525 CrossRef CAS.
- S. Kutsumizu, Isr. J. Chem., 2012, 52, 844 CrossRef CAS.
- B.-K. Cho, RSC Adv., 2014, 4, 395 RSC.
- X. Feng, M. E. Tousley, M. G. Cowan, B. R. Wiesenauer, S. Nejati, Y. Choo, R. D. Noble, M. Elimelech, D. L. Gin and C. O. Osuji, ACS Nano, 2014, 8, 11977 CrossRef CAS PubMed.
- B. Soberats, E. Uchida, M. Yoshio, J. Kagimoto, H. Ohno and T. Kato, J. Am. Chem. Soc., 2014, 136, 9552 CrossRef CAS PubMed.
- B. Soberats, M. Yoshio, T. Ichikawa, H. Ohno and T. Kato, J. Mater. Chem. A, 2015, 3, 11232 CAS.
- Y. Wang, K. S. Chen, J. Mishler, S. C. Cho and X. C. Adroher, Appl. Energy, 2011, 88, 981 CrossRef CAS.
- R. D. Costa, F. Werner, X. J. Wang, P. Gronninger, S. Feihl, F. T. U. Kohler, P. Wasserscheid, S. Hibler, R. Beranek, K. Meyer and D. M. Guldi, Adv. Energy Mater., 2013, 3, 657 CrossRef CAS.
- J. Sakuda, E. Hosono, M. Yoshio, T. Ichikawa, T. Matsumoto, H. Ohno, H. Zhou and T. Kato, Adv. Funct. Mater., 2015, 25, 1206 CrossRef CAS.
- T. Ichikawa, M. Yoshio, A. Hamasaki, S. Taguchi, F. Liu, X. B. Zeng, G. Ungar, H. Ohno and T. Kato, J. Am. Chem. Soc., 2012, 134, 2634 CrossRef CAS PubMed.
- C. Tschierske, Angew. Chem., Int. Ed., 2013, 52, 8828 CrossRef CAS PubMed.
- L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071 CrossRef CAS PubMed.
- C. R. South, C. Burd and M. Weck, Acc. Chem. Res., 2007, 40, 63 CrossRef CAS PubMed.
- S. Seiffert and J. Sprakel, Chem. Soc. Rev., 2012, 41, 909 RSC.
- F. Huang and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 5879 RSC.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813 CrossRef CAS PubMed.
- L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196 CrossRef CAS PubMed.
- C. C. Cheng, F. C. Chang, J. H. Wang, Y. L. Chu, Y. S. Wang, D. J. Lee, W. T. Chuang and Z. Xin, RSC Adv., 2015, 5, 76451 RSC.
- C. C. Cheng, F. C. Chang, J. H. Wang, J. K. Chen, Y. C. Yen and D. J. Lee, Nanoscale, 2016, 8, 723 RSC.
- C. C. Cheng, F. C. Chang, J. K. Chen, T. Y. Wang and D. J. Lee, RSC Adv., 2015, 5, 101148 RSC.
- S. D. Brown and S. G. Greenbaum, Solid State Ion., 1994, 67, 257 CrossRef CAS.
- C. C. Cheng, C. F. Huang, Y. C. Yen and F. C. Chang, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6416 CrossRef CAS.
- C. C. Cheng, Y. C. Yen, Y. S. Ye and F. C. Chang, J. Polym. Sci., Part A: Polym. Chem., 2009, 46, 6388 CrossRef.
- C. Y. Chiu, H. W. Chen, S. W. Kuo, C. F. Huang and F. C. Chang, Macromolecules, 2004, 37, 8424 CrossRef CAS.
- J. H. Wang, C. C. Cheng, O. Altukhov, F. C. Chang and S. W. Kuo, Polymers, 2013, 5, 937 CrossRef.
- (a) V. G. H. Lafitte, A. E. Aliev, P. N. Horton, M. B. Hursthouse, K. Bala, P. Golding and H. C. Hailes, J. Am. Chem. Soc., 2006, 128, 6544 CrossRef CAS PubMed; (b) V. G. H. Lafitte, A. E. Aliev, E. Greco, K. Bala, P. Golding and H. C. Hailes, New J. Chem., 2011, 35, 1522 RSC.
- M.-L. Saboungi, D. L. Price, G. Mao, R. Fernandez-Perea, O. Borodin, G. D. Smith, M. Armand and W. S. Howells, Solid State Ionics, 2002, 147, 225 CrossRef CAS.
- J. Sun, G. M. Stone, N. P. Balsara and R. N. Zuckermann, Macromolecules, 2012, 45, 5151 CrossRef CAS.
- I. Honma and M. Yamada, Bull. Chem. Soc. Jpn., 2007, 80, 2110 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The detailed synthetic procedures and instrumentation used in this research are described in more detail in ESI. See DOI: 10.1039/c6ra07011f |
| This journal is © The Royal Society of Chemistry 2016 |

