Golf ball-like architecture fabricated by supramolecular self-assembly of naphthalene diimide†
Santosh P. Goskulwadab,
Duong Duc Lac,
Rajesh S. Bhosalea,
Mohammad Al Kobaisic,
Sheshanath V. Bhosale*c and
Sidhanath V. Bhosale*a
aPolymers and Functional Materials Division, CSIR-Indian Institute of Chemical Technology, Hyderabad-500007, Telangana, India. E-mail: bhosale@iict.res.in
bAcademy of Scientific and Innovative Research (AcSIR), CSIR-IICT, Hyderabad-500007, Telangana, India
cSchool of Applied Sciences, RMIT University, GPO Box 2476, Melbourne, VIC-3001, Australia. E-mail: Sheshanath.bhosale@rmit.edu.au
First published on 13th April 2016
Abstract
In this manuscript we demonstrate a simple procedure for the fabrication of wrinkled surface golf ball-like nanostructures in a 3D fashion using naphthalene diimide (NDI-S) derivatives. The golf ball-like microsphere morphology was obtained by mixing NDI-S in 45% hexane with a CHCl3 solution. The structural formation was visualised by scanning electron microscopy (SEM), transmission electron microscopy (TEM), while DLS, UV-vis absorption and fluorescence were used to determine the mode of aggregation.
Supramolecular self-assembled materials are of considerable interest due to their promising applications in optoelectronics, chemosensors, nanotechnology, biotechnology and biomedicines.1–3 Controlled supramolecular structures such as nanowires, nanoribbons, nanotubes, nano/micro-belts, nanosheets, and nano/micro-flowers from small aromatic π-conjugated functional molecules have even more promising properties and potential applications.4–10 Currently several methods exist for the synthesis and preparation of supramolecular assemblies and their sub-units. This offers controlled self-assembly methods for fabricating versatile soft materials from simple organic molecules. Amongst the reported self-assembly methods stimuli response is one of the most promising methods in the supramolecular chemistry and self-assembly. Hydrogen bonding mediated supramolecular self-assemblies of small aromatic π-conjugated systems has been investigated for the construction of nano/micro-meter functional materials.11–13
In recent years continuous efforts have been focused on the fabrication of colloidal particle assemblies of the organic molecules because of their potential applicability in the field of photocatalysis, superhydrophobic (non-wetting) materials for water treatment, toxic ion removal, trace detection biosensors, environmental gas sensing, explosives detection materials, optoelectronic materials as well as their biological and biomedical applications.14 However, a only few examples illustrate formation of golf ball-like structures from conjugated aromatic molecules and/or polymers.15–22 Furthermore, the fabrication of such golf ball-like nanostructures often requires complicated procedures that change the smooth surface appearance of spheres to wrinkled appearance and are infancy and not yet fully explored in terms of organic functional molecules.
Among aromatic molecules, naphthalene diimide (NDI) have been used in many areas such as supramolecular chemistry, solar cell, medicinal chemistry, ion channel and material chemistry due to their excellent n-type conductivity and has been widely studied in organic electronics.23,24 The fabrication of various nanostructures such as nanotubes, nanobelts, nanoparticles, organogels, hydrogels, and synthetic ion channels was achieved via supramolecular self-assembly based on NDIs.25–30 Recently, we have reported self-assembly of NDI amphiphile and bolaamphiphile into variety of nanostructures such as well-defined interwoven fibres, ladder-type network, complex fractal nanostructures, nanobelts, vesicles, chiral assembly, worm-like structures, nanoparticles and microflowers.31–35 Although the previous methods successfully employed to construct various complex nanostructures, a method for fabrication of golf-ball-shaped microspheres having wrinkles on their surface, based on NDI, has not yet been reported. Therefore, developing a supramolecular self-assembly method to fabricate golf ball-like microspheres remains a challenge and is significant both in fundamental and applied research.
Herein, we demonstrate a simple method based on supramolecular self-assembly of NDI bearing dipeptide at one end of imide position (NDI-S) and amine head group at the other end (Fig. 1) for the preparation of uniform golf ball-like microspheres with wrinkled surfaces which require no post treatment. The supramolecular golf-ball like architectures described herein is the first to be formed from small π-conjugated molecule. In this case, NDI-S possesses three important features that result in the formation of controlled golf ball-like morphology: (i) the core of the NDI which allows molecule to arrange through π–π interactions and (ii) the amine functionality at one end of imide interacts with another molecule via strong hydrogen-bonding (H-bonding) and (iii) the hydrophobic ester on the other side of NDI interact with another molecule through van der Waals forces. We believe such special amphiphilic structures of NDI-S, having both hydrophilic and hydrophobic group, prevent the crystallisation and direct the golf ball-like morphology as illustrated in Fig. 1.
The detail synthesis of NDI-S is outlined in ESI.†36 The supramolecular self-assembly of NDI-S was studied in CHCl3/hexane (0–50%) mixture. The UV-vis absorption of NDI-S (1 × 10−5 M) in CHCl3 showed two well resolved absorption peaks at 370 nm and 355 nm along with a small shoulder at 347 nm, which is characteristic of the S0 → S1 transition attributed to π–π* transition (Fig. 2a). The UV-vis absorption spectra of NDI-S significantly changed in the higher concentration of hexane (0–50%) in CHCl3. Fig. 2a clearly shows that increasing hexane fraction a reduction of absorption peak intensities with significant red shifts. These results demonstrate that under the influence of a non-polar solvent, resulted supramolecular self-organisation of NDI-S, similar J-type of aggregates shown for other examples.37,38
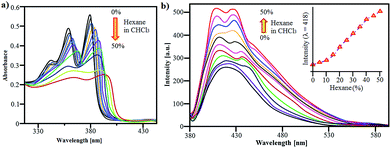 | ||
| Fig. 2 Solution based self-assembly. (a) & (b), UV-vis absorption and emission changes of NDI-S (1 × 10−5 M) in CHCl3/hexane (0–50%), respectively. | ||
The fluorescence emission spectra of NDI-S in CHCl3 showed well resolved peak at 425 nm (λex = 365 nm) (Fig. 2b). Interestingly, the emission of NDI-S increases with two distinct peaks with maxima at 400 nm and 438 nm with increasing percentage of hexane as shows in Fig. 2b. The fluorescence spectroscopy results clearly suggest that NDI-S exhibits strong fluorescence emission upon aggregation such effect so called aggregation induced emission enhancement (AIEE).39,40
The absorption and emission spectroscopy results attributed to the formation of aggregates via H-bonding, face-to-face π-stacks of NDI chromophores and van der Waals interactions. To gain further insight into morphology of NDI-S, Field Emission Scanning Electron Microscopy (FE-SEM) technique was employed. A solution of NDI-S (1 × 10−4 M) was prepared in CHCl3/hexane (fh = 45%) and allowed to reach equilibrium. FE-SEM measurements were performed by drop-casting on silicon wafer substrate and then were air dried. Fig. 3(A) and (B) images showing golf ball-like microspheres with a wrinkled surface with an average in several micrometers diameter. Fig. 3B is the 3D hierarchical magnifying image of golf balls morphology of NDI-S with wrinkled surface. SEM images clearly showed that the golf-ball like microspheres consists of wrinkled surface along with some dents (ESI Fig. S21†). We presume that the dent formation may be due to release of solvent from the microspheres.19–21 The microspheres formed by the aggregation of NDI-S to form cores of the microspheres followed by further growth up to 1–2 μm. It appears that the aging of these spherical aggregates result in smaller and larger particle than the initial ones. The smooth surface changes to wrinkle at the last stage because of instability in thermodynamics due to hydrophobicity difference of the two head groups of NDI-S like a golf ball.
 | ||
| Fig. 3 SEM micrographs of NDI-S in CHCl3/hexane (45%, v/v): (A) microspheres and (B) showing the high magnification image of the golf ball-like microsphere with wrinkled surface by Ostwald ripening process.41 | ||
To understand the mode of aggregation in self-assembly through the solvophobic effect, transmission electron microscopy (TEM) was performed. TEM analysis clearly shows well-defined spherical aggregates with varying sizes with range from 250 nm to 1.5 μM from CHCl3/hexane (45%, v/v) as shown in Fig. 4a. Initially, the smaller spheres (∼250–400 nm) formed by the aggregation of NDI-S followed by further growth up to 1–2 μm in size. TEM image (inset of Fig. 4a) clearly shows the core and shell of the particular aggregates, we believe such core-curvature seen in TEM analysis is due to wrinkle morphology on to the surface of the microsphere. Furthermore, dynamic laser scattering (DLS) measurements confirmed the formation of spherical aggregates in CHCl3/hexane (fh = 45%) solution (Fig. 4b). The NDI-S gives an average sizes (hydrodynamic sizes) of 1300 nm and 350 nm. The size of the NDI-S aggregates in DLS is similar to that of golf ball superstructures measured using SEM and TEM microscopy.
Density functional theory (DFT) calculations using Gaussian 09 suite of programs and geometry optimization at B3LYP/6-311G level of theory of the NDI-S molecule gave a HOMO → LUMO gap of (4.796 eV) 258.5 nm.42 The electron density of the HOMO, HOMO−1 and HOMO−2 are concentrated on the benzene ring moiety of the molecule structure, and the LUMO, LUMO+1, and LUMO+2 are concentrated on the NDI core constituting the electron acceptor moiety (see Fig. 5 and ESI S22†).
In summary, a supramolecular self-assembly method was developed to fabricate golf ball-like microstructures with wrinkled surface in organic solvents. The formation of golf ball-like microstructures have been well characterised by SEM and DLS analysis. The hydrogen bonding along with π-stacking is the driving force for formation of spherical structures. We believe that the amine functional groups at one imide end of NDI-S play an important role to construct golf ball-like structures with wrinkled surface. The development of this simple method of fabricating golf ball-like microspheres may stimulate further advances in supramolecular self-assembly theory with potential applications in material and biomedical applications. Furthermore, NDI-S induce enhancement of emission upon aggregation which could be used as a potential fluorescent material within sensors.
Acknowledgements
S. V. B. (IICT) is grateful for financial support from the DAE-BRNS (Project Code: 37(2)/14/08/2014-BRNS), Mumbai, and Intelcoat project CSC0114, CSIR, New Delhi, India. S. G. acknowledges financial support from UGC, New Delhi for SRF fellowship. R. S. B. acknowledges financial support from CSIR, New Delhi under the SRA scheme [(13(8772)-A)/2015-Pool]. S. V. B. (RMIT) acknowledges financial support from the Australian Research Council under a Future Fellowship Scheme (FT110100152). The authors acknowledge the facilities, and the scientific and technical assistance, of the Australian Microscopy & Microanalysis Research Facility at RMIT University.Notes and references
- J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, Wiley-VCH, Weinheim, 1995 Search PubMed.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef CAS PubMed.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- S. S. Babu, H. Möhwald and T. Nakanishi, Chem. Soc. Rev., 2010, 39, 4021–4035 RSC.
- R. Charvet, Y. Yamamoto, T. Sasaki, J. Kim, K. Kato, M. Takata, A. Saeki, S. Seki and T. Aida, J. Am. Chem. Soc., 2012, 134, 2524–2527 CrossRef CAS PubMed.
- Y. Yamamoto, T. Fukushima, Y. Suna, N. Ishii, A. Saeki, S. Seki, S. Tagawa, M. Taniguchi, T. Kawai and T. Aida, Science, 2006, 314, 1761–1764 CrossRef CAS PubMed.
- W. Jiang, Y. Zhou, H. Geng, S. Jiang, S. Yan, W. Hu, Z. Wang, Z. Shuai and J. Pei, J. Am. Chem. Soc., 2011, 133, 1–3 CrossRef CAS PubMed.
- F. Würthner, C. R. Saha-Möller, B. Fimmel, S. Ogi, P. Leowanawat and D. Schmidt, Chem. Rev., 2016, 116, 962–1052 CrossRef PubMed.
- S. V. Bhosale, S. V. Bhosale and S. K. Bhargava, Org. Biomol. Chem., 2012, 10, 6455–6468 CAS.
- S. V. Bhosale, C. H. Jania and S. J. Langford, Chem. Soc. Rev., 2008, 37, 331–342 RSC.
- S. Yagai, Bull. Chem. Soc. Jpn., 2015, 88, 28–58 CrossRef.
- B. Roy, P. Bairi and A. K. Nandi, RSC Adv., 2014, 4, 1708–1734 RSC.
- F. Würthner, Chem. Commun., 2004, 1564–1579 RSC.
- Z. Ren, Y. Guo, C.-H. Liu and P.-X. Gao, Front. Chem., 2013, 1, 1–22 CAS.
- A. P. H. J. Schenning, B. De Bruin, M. C. Feiters and R. J. M. Nolte, Angew. Chem., Int. Ed. Engl., 1994, 33, 1662–1663 CrossRef.
- M. Okubo, Y. Murakami and T. Fujiwara, Colloid Polym. Sci., 1996, 274, 520–524 CAS.
- M. Okubo, A. Yamaguchi and T. Fujiwara, Colloid Polym. Sci., 1999, 277, 1005–1008 CAS.
- T. Fujibayashi, Y. Komatsu, N. Konishi, H. Yamori and M. Okubo, Ind. Eng. Chem. Res., 2008, 47, 6445–6449 CrossRef CAS.
- H. Maeda, M. Hasegawa, T. Hashimoto, T. Kakimoto, S. Nishio and T. Nakanishi, J. Am. Chem. Soc., 2006, 128, 10024–10025 CrossRef CAS PubMed.
- K.-H. Hwangbo, M. R. Kim, C.-S. Lee and K. Y. Cho, Soft Matter, 2011, 7, 10874–10878 RSC.
- Q. Zhou, H. Xiang, H. Fan, X. Yang, N. Zhao and J. Xu, J. Mater. Chem., 2011, 21, 13056–13061 RSC.
- J. H. Lee, C.-S. Lee and K. Y. Cho, ACS Appl. Mater. Interfaces, 2014, 6, 16493–16497 CAS.
- S. L. Suraru and F. Würthner, Angew. Chem., Int. Ed. Engl., 2014, 53, 7428–7448 CrossRef CAS PubMed.
- R. Bhosale, J. Misek, N. Sakai and S. Matile, Chem. Soc. Rev., 2010, 39, 138–149 RSC.
- N. Sakai, J. Mareda, E. Vauthey and S. Matile, Chem. Commun., 2010, 46, 4225–4237 RSC.
- H. Shao and J. R. Parquette, Chem. Commun., 2010, 46, 4285–4287 RSC.
- S. V. Bhosale, C. H. Jani, C. H. Lalander, S. J. Langford, I. Nerush, J. G. Shapter, D. Villamaina and E. Vauthey, Chem. Commun., 2011, 47, 8226–8228 RSC.
- Q. Meng and W. Hu, Phys. Chem. Chem. Phys., 2012, 14, 14152–14164 RSC.
- K. Liu, Y. Yao, Y. Liu, C. Wang, Z. Li and X. Zhang, Langmuir, 2012, 28, 10697–10702 CrossRef CAS PubMed.
- S. V. Bhosale, M. Adsul, G. V. Shitre, S. R. Bobe, S. V. Bhosale and S. H. Priver, Chem.–Eur. J, 2013, 19, 7310–7313 CrossRef CAS PubMed.
- S. V. Bhosale, C. H. Jani, C. H. Lalander, S. J. Langford, I. Nerush, J. G. Shapter, D. Villamaina and E. Vauthey, Chem. Commun., 2011, 47, 8226–8228 RSC.
- S. V. Bhosale, C. Jani, C. H. Lalander and S. J. Langford, Chem. Commun., 2010, 46, 973–975 RSC.
- K. P. Nandre, M. Al Kobaisi, R. S. Bhosale, K. Latham, S. V. Bhosale and S. V. Bhosale, RSC Adv., 2014, 4, 40381–40384 RSC.
- K. P. Nandre, S. V. Bhosale, K. V. S. Rama Krishna, A. Gupta and S. V. Bhosale, Chem. Commun., 2013, 49, 5444–5446 RSC.
- R. S. Bhosale, M. Al Kobaisi, S. V. Bhosale, S. Bhargava and S. V. Bhosale, Sci. Rep., 2015, 5, 14609 CrossRef CAS PubMed.
- M. Pandeeswar, H. Khare, S. Ramakumar and T. Govindaraju, RSC Adv., 2014, 4, 20154–20163 RSC.
- S. V. Bhosale, N. V. Ghule, M. A. Kobaisi, M. M. A. Kelson and S. V. Bhosale, Chem.–Eur. J., 2014, 20, 10775–10781 CrossRef CAS PubMed.
- N. V. Ghule, D. Duc La, R. S. Bhosale, M. A. Kobaisi, A. M. Raynor, S. V. Bhosale and S. V. Bhosale, ChemistryOpen, 2016 DOI:10.1002/open.201500201.
- A. Rananaware, D. D. La and S. V. Bhosale, RSC Adv., 2015, 5, 63130–63134 RSC.
- A. Rananaware, D. D. La, S. M. Jackson and S. V. Bhosale, RSC Adv., 2016, 6, 16250–16255 RSC.
- P. Hu, L. Yu, A. Zuo, C. Guo and F. Yuan, J. Phys. Chem. C, 2009, 113, 900–906 CAS.
- M. J. Frischet al., Gaussian 09, revision D.01, Gaussian, Inc., Wallingford CT, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures, detail experimental protocol, full characterisation and spectroscopic data of all new compounds. See DOI: 10.1039/c6ra06927d |
| This journal is © The Royal Society of Chemistry 2016 |



