Removal of heavy metal ions using functionalized graphene membranes: a molecular dynamics study
Abstract
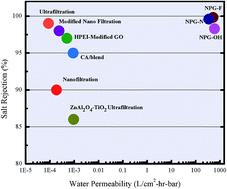
Industrial wastewater contains toxic metals such as copper, lead, cadmium, zinc and cobalt. Hence, it is necessary to eliminate the toxic heavy metal ions from wastewater before it is released into the environment. In this study, we have used classical molecular dynamics (MD) simulations and density functional theory (DFT) calculations to investigate the desalination performance of nanoporous graphene (NPG) membranes for different pore sizes and chemical functionalization (hydroxyl, nitrogen and fluorine) of the pore. The underlying mechanism involved in the separation process is explained using potential of mean force (PMF) calculations and plane-wave (PW) DFT calculations. The estimated energy barriers using DFT calculations were found to be in good agreement with the results obtained using classical MD simulations. This study shows that the NPG functionalized with N (NPG-N) shows higher salt rejection with intermediate permeability compared NPG functionalized with F (NPG-F) and OH (NPG-OH). NPG-OH shows higher water permeability with lower salt rejection compared to NPG-N and NPG-F. However, NPG-F shows lowest permeability compared to other two NPGs considered in this study. Even at high pressures like 500 MPa the salt rejection percentage is not less than 90% and the minimum permeability is 270 L cm−2 h−1 bar−1. Overall, our results indicate that the water permeability of NPG membranes is about 4–5 orders of magnitude higher than the existing technologies. Thus, the NPG membrane may have a valuable role to play in industrial waste water purification.

 Please wait while we load your content...
Please wait while we load your content...