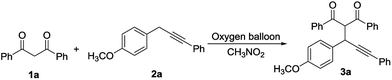
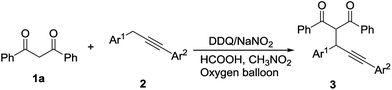
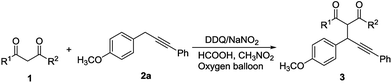
Propargylation of 1,3-dicarbonyl compounds catalyzed by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone and sodium nitrite in the presence of molecular oxygen and formic acid†
Dongping Cheng*a,
Xiayi Zhoua,
Xiaoliang Xub and
Jizhong Yana
aCollege of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310014, P. R. China. E-mail: chengdp@zjut.edu.cn
bCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, P. R. China
First published on 24th May 2016
Abstract
Catalyzed by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and sodium nitrite, the coupling reaction of 1,3-diarylpropynes and 1,3-dicarbonyl compounds using molecular oxygen as a terminal oxidant was developed. The reaction was promoted dramatically in the presence of HCOOH. The corresponding products were obtained within half an hour in 37–87% yields.
C–C bond formation is one of the most useful and fundamental processes in organic synthesis. In recent years, the cross-dehydrogenative coupling reaction (CDC reaction) of activated C–H bonds has become an attractive research topic.1,2 From the point of green chemistry, molecular oxygen is an ideal oxidant in the CDC transformation due to its naturalness, inexpensiveness, and water being the sole by-product. It is well known that, for fulfilling this kind of transformation, a catalyst is necessary to overcome the barrier between the triplet ground state of molecular oxygen and the singlet ground state of common substrates. Great progress has been made in the development of excellent catalysts to achieve this goal.3–5 However, the direct C–C coupling from Csp3–H bonds with molecular oxygen as the terminal oxidant is still a challenging task for organic chemists.
Propargylation of 1,3-dicarbonyl compounds is an important C–C bond forming method in organic synthesis because the alkyne and carbonyl group act as precursors of different functional groups. For example, the reaction of propargyl-substituted 1,3-dicarbonyl compound with phenylhydrazine hydrochloride affords indazole which could be applied as a further synthetic reagent for synthesizing pharmacologically active compounds.6 The condensation of propargyl-substituted 1,3-dicarbonyl compound with amine gives pyrrole which is a common subsection in many pharmaceuticals.7,8 Sanz and others reported that propargyl-substituted 1,3-dicarbonyl compounds could be transformed to substituted furans that represent important building blocks in many pharmaceuticals, flavours and fragrance compounds.8–11 DDQ as an effective oxidant has been widely used for cross-dehydrogenative coupling.12–15 Recently, our group reported the coupling reaction of allylic compounds and 1,3-dicarbonyls catalyzed by DDQ/NaNO2 with molecular oxygen as the terminal oxidant.16 With HCOOH as an additive, unexpectedly, the reaction was promoted dramatically. To explore further the role of HCOOH and according to the similar reactivity between propargylic and allylic compound, we tried to apply the catalytic system for the reaction of propargylic compounds with 1,3-dicarbonyls. Compared with the benzylation and allylation of 1,3-dicarbonyl compounds, the propargylation was little studied.17–37 Although some results were achieved in the propargylation of 1,3-dicarbonyl compounds, a good choice for the transformation through the cross-dehydrogenative coupling with molecular oxygen as the terminal oxidant has not been reported. With the interest of the application of the oxidative system of DDQ/O2 in organic synthesis, herein, we report the propargylation of 1,3-dicarbonyl compounds catalyzed by DDQ and NaNO2 in the presence of molecular oxygen and HCOOH.
The coupling of 1,3-diphenyl-1,3-propanedione 1a and 1-phenyl-3-(4-methoxyl)phenyl-1-propyne 2a was chosen as a model reaction (Table 1). Referred to the optimized reaction condition of allylic compounds and 1,3-dicarbonyls,16 it was catalyzed by 1 mol% DDQ and 10 mol% NaNO2 in the presence of oxygen balloon and HCOOH. To our delight, the expected coupling product was obtained with 65% yield (entry 1). The yield was promoted to 82% when the catalytic amount of DDQ was 10 mol% (entry 2). The reaction was completed within half an hour. It was noteworthy that the reaction which was mediated by a stoichiometric of DDQ without additional reagent, need three hours to complete.24 To our surprise, the coupling reaction could be performed only in the presence of HCOOH and CH3NO2 and give the coupling product with 17% yield (entry 3). However no expected coupling product was obtained when allylic compounds reacted with 1,3-dicarbonyls by HCOOH/CH3NO2.38 It indicated that the mechanism of the coupling reaction should be different from the coupling reaction of allylic compounds and 1,3-dicarbonyls. That was to say that the C–C formation could be mediated not only by DDQ. Another acid such as PhCOOH, CF3COOH, and CH3COOH was surveyed, while no coupling product was obtained. To probe further the possible mechanism, some control experiments were performed. The coupling product was obtained in 76% yield when DDQH2 (10 mol%) took place of DDQ in the reaction (entry 4). It indicated that DDQH2 could regenerate DDQ by NaNO2/HCOOH/O2. In the absence of NaNO2, DDQ, or HCOOH, the yield was decreased to 23%, 20% and 15%, respectively (entries 5–7). It gave the product with 35% yield when the reaction was performed in the presence of nitrogen instead of molecular oxygen (entry 8). No product was obtained when the reaction was performed only by NaNO2/O2 (entry 9). The yield of coupling product was decreased dramatically when TEMPO was added to the mixture (entry 12). It showed that the reaction might proceed through radical process. Based on the above results, it could be concluded that DDQ and HCOOH mediated the coupling reaction respectively. However the role of HCOOH was unclear.
| Entry | DDQ (mol%) | NaNO2 (mol%) | HCOOH (mL) | Yieldb (%) |
|---|---|---|---|---|
| a 1a (0.5 mmol), 2a (0.6 mmol), DDQ, NaNO2, HCOOH, CH3NO2 (2.5 mL), 0.5 h, r.t.b Isolation yield.c DDQH2 (10 mol%) instead of DDQ.d Nitrogen instead of molecular oxygen.e TEMPO (0.5 mmol) was added to the mixture. | ||||
| 1 | 1 | 10 | 0.25 | 65 |
| 2 | 10 | 10 | 0.25 | 82 |
| 3 | — | — | 0.25 | 17 |
| 4c | — | 10 | 0.25 | 76 |
| 5 | 10 | — | 0.25 | 23 |
| 6 | — | 10 | 0.25 | 20 |
| 7 | 10 | 10 | — | 15 |
| 8d | 10 | 10 | 0.25 | 35 |
| 9 | — | 10 | — | 0 |
| 10d | — | — | 0.25 | 16 |
| 11 | 10 | — | — | 16 |
| 12e | 10 | 10 | 0.25 | 18 |
To obtain the different substrate effect on the reaction, the coupling reaction of 1,3-diarylpropyne 2 with 1,3-diphenyl-1,3-propanedione 1a was firstly investigated. From Table 2, the group on benzyl ring of compound 2 had obvious effect on the reaction. The yield was higher relatively when the group such as methyl and methoxyl was donating electron (entries 1–4). The yield was lower when the group was chloro (entry 5). It indicated that the reaction might generate the propargyl cation and/or radical intermediacy. The donating electron group was in favor of the stability of intermediacy and facilitated the formation of coupling product. The methoxyl group of benzene ring adjacent to alkynyl had little effect on the reaction (entry 6).
Next, a series of 1,3-propanedione was subject to the reaction and results were listed in Table 3. It gave the coupling product with 68–85% yield when 1,3-propanedione 1, which contained electron-donating substitute such as methyl, methoxyl on the benzene ring, reacted with 1-phenyl-3-(4-methoxyl) phenyl-1-propyne 2a (entries 1–4). The yield was relatively lower when 1,3-propanedione 1 bearing electron-withdrawing substitute such as chloro and bromo on the benzene ring was used as the substrates (entries 5–6). Heterocyclic aryl groups such as furanyl and thienyl were good candidates in the reaction and gave the products in 85–87% yields (entries 7–8). Benzoylacetone was a suitable partner here (entry 9). Acetylacetone and ethyl benzoylacetate could be also applied despite the slightly lower yields (entries 10–11).
| Entry | R1 | R2 | 3 | Yieldb (%) |
|---|---|---|---|---|
| a 1 (0.5 mmol), 2a (0.6 mmol), DDQ (10 mol%), NaNO2 (10 mol%), HCOOH (0.25 mL), CH3NO2 (2.5 mL), 0.5 h, r.t.b Isolation yield. | ||||
| 1 | 4-CH3C6H4 | C6H5 | 3g | 85 |
| 2 | 4-CH3OC6H4 | C6H5 | 3h | 75 |
| 3 | 3-CH3C6H4 | C6H5 | 3i | 68 |
| 4 | 2-CH3C6H4 | C6H5 | 3j | 68 |
| 5 | 4-ClC6H4 | C6H5 | 3k | 46 |
| 6 | 4-BrC6H4 | C6H5 | 3l | 48 |
| 7 | 2-Thienyl | C6H5 | 3m | 87 |
| 8 | 2-Furyl | C6H5 | 3n | 85 |
| 9 | CH3 | C6H5 | 3o | 73 |
| 10 | CH3 | CH3 | 3p | 53 |
| 11 | OCH2CH3 | C6H5 | 3q | 49 |
We have developed an efficient propargylation of 1,3-dicarbonyl compounds catalyzed by DDQ and NaNO2 with molecular oxygen as a terminal oxidant. It promoted dramatically in the presence of HCOOH. However the mechanism of the reaction was unclear. The reaction system will be continued to explore further in our lab.
Experimental
Column chromatography was carried out on silica gel (200–300 mesh). 1H NMR spectra were recorded on a 500 MHz spectrometer. 13C NMR spectra were recorded on a 125 MHz spectrometer. Chemical shifts were reported in parts per million (δ) relative to the internal standard TMS (0 ppm) for CDCl3. The coupling constants, J, are reported in Hertz (Hz). High-resolution mass spectra (HRMS) were recorded on ESI-TOF. Melting points were uncorrected. The reagents were purchased from commercial chemical reagent companies and used without further purification unless otherwise stated.General procedure
To a mixture of 1,3-dicarbonyl compound 1 (0.5 mmol) and 1,3-diarylpropyne 2 (0.6 mmol) in CH3NO2 (2.5 mL), DDQ (11.3 mg, 0.05 mmol), NaNO2 (3.5 mg, 0.05 mmol), HCOOH (0.25 mL) were added. It was carried out at room temperature in the presence of oxygen atmosphere (oxygen balloon) within half an hour. The resulting mixture was concentrated under reduced pressure and purified by flash column chromatography on silica gel (petroleum ether–ethyl acetate = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–20
1–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the desired pure product 3.
1) to give the desired pure product 3.
Acknowledgements
This work was supported by Natural Science Foundation of Zhejiang Provincial (LY15B020004) and the Open Research Fund Program of Collaborative Innovation Center of Membrane Separation and Water Treatment (2016YB07).Notes and references
- C. J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed.
- C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed.
- T. Punniyamurthy, S. Velusamy and J. Iqbal, Chem. Rev., 2005, 105, 2329 CrossRef CAS PubMed.
- D. Lenoir, Angew. Chem., Int. Ed., 2006, 45, 3206 CrossRef CAS PubMed.
- M. S. Sigman and D. R. Jensen, Acc. Chem. Res., 2006, 39, 221 CrossRef CAS PubMed.
- J. W. Lim, K. H. Kim, S. H. Kim and J. N. Kim, Tetrahedron, 2014, 70, 6831 CrossRef CAS.
- X. T. Liu, L. Huang, F. J. Zheng and Z. P. Zhan, Adv. Synth. Catal., 2008, 350, 2778 CrossRef CAS.
- P. N. Chatterjee and S. Roy, Tetrahedron, 2011, 67, 4569 CrossRef CAS.
- R. Sanz, D. Miguel, A. Martínez, J. M. Álvarez-Gutiérrez and F. Rodríguez, Org. Lett., 2007, 9, 727 CrossRef CAS PubMed.
- V. Cadierno, J. Gimeno and N. Nebra, Adv. Synth. Catal., 2007, 349, 382 CrossRef CAS.
- X. Feng, Z. Tan, D. Chen, Y. Shen, C. C. Guo, J. Xiang and C. Zhu, Tetrahedron Lett., 2008, 49, 4110 CrossRef CAS.
- R. Samanta, K. Matcha and A. P. Antonchick, Eur. J. Org. Chem., 2013, 5769 CrossRef CAS.
- S. A. Girard, T. Knauber and C. J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef CAS PubMed.
- C. Liu, D. Liu and A. Lei, Acc. Chem. Res., 2014, 47, 3459 CrossRef CAS PubMed.
- I. B. Krylov, V. A. Vil and A. O. Terent'ev, Beilstein J. Org. Chem., 2015, 11, 92 CrossRef PubMed.
- D. Cheng, K. Yuan, X. Zhou and J. Yan, Tetrahedron Lett., 2015, 56, 1641 CrossRef CAS.
- X. Yao and C. J. Li, J. Org. Chem., 2005, 70, 5752 CrossRef CAS PubMed.
- Z. Li and C. J. Li, J. Am. Chem. Soc., 2006, 128, 56 CrossRef CAS PubMed.
- Y. Zhang and C. J. Li, Angew. Chem., Int. Ed., 2006, 45, 1949 CrossRef CAS PubMed.
- Z. Li, L. Cao and C. J. Li, Angew. Chem., Int. Ed., 2007, 46, 6505 CrossRef CAS PubMed.
- O. Baslé and C. J. Li, Green Chem., 2007, 9, 1047 RSC.
- Z. Li, R. Yu and H. Li, Angew. Chem., Int. Ed., 2008, 47, 7497 CrossRef CAS PubMed.
- N. Borduas and D. A. Powell, J. Org. Chem., 2008, 73, 7822 CrossRef CAS PubMed.
- D. Cheng and W. Bao, J. Org. Chem., 2008, 73, 6881 CrossRef CAS PubMed.
- D. Cheng and W. Bao, Adv. Synth. Catal., 2008, 350, 1263 CrossRef CAS.
- M. F. Ali, R. Çalışkan, E. Şahin and M. Balci, Tetrahedron, 2009, 65, 1430 CrossRef CAS.
- C. A. Correia and C. J. Li, Tetrahedron Lett., 2010, 51, 1172 CrossRef CAS.
- D. Ramesh, U. Ramulu, S. Rajaram, P. Prabhakar and Y. Venkateswarlu, Tetrahedron Lett., 2010, 51, 4898 CrossRef CAS.
- C. G. Piscopo, S. Bühler, G. Sartori and R. Maggi, Catal. Sci. Technol., 2012, 2, 2449 CAS.
- D. Cheng, X. Ye, W. Cui, R. Sun and J. Yan, J. Chem. Res., 2012, 36, 729 CrossRef CAS.
- S. Pan, J. Liu, Y. Li and Z. Li, Chin. Sci. Bull., 2012, 57, 2382 CrossRef CAS.
- Á. Pintér and M. Klussmann, Adv. Synth. Catal., 2012, 354, 701 CrossRef.
- D. Cheng, K. Yuan, X. Zhou and J. Yan, J. Chem. Res., 2014, 38, 751 CrossRef CAS.
- M. Xiang, Q. Y. Meng, J. X. Li, Y. W. Zheng, C. Ye, Z. J. Li, B. Chen, C. H. Tung and L. Z. Wu, Chem.–Eur. J., 2015, 21, 18080 CrossRef CAS PubMed.
- F. Li, Z. Meng, J. Hua, W. Li, H. Lou and L. Liu, Org. Biomol. Chem., 2015, 13, 5710 CAS.
- K. Yang and Q. Song, Org. Lett., 2015, 17, 548 CrossRef CAS PubMed.
- M. Xiang, Q. Y. Meng, X. W. Gao, T. Lei, B. Chen, C. H Tung and L. Z. Wu, Org. Chem. Front., 2016, 3, 486 RSC.
- To a mixture of 1,3-diphenylpropene (0.6 mmol), 1,3-diphenyl-1,3-propanedione (0.5 mmol) in CH3NO2 (2.5 mL), HCOOH (0.25 mL) was added. No coupling product was isolated within half an hour or longer.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra of compounds 3. See DOI: 10.1039/c6ra06704b |
| This journal is © The Royal Society of Chemistry 2016 |