Polyaminocyclodextrin nanosponges: synthesis, characterization and pH-responsive sequestration abilities
Marco Russoa,
Maria Luisa Saladinoab,
Delia Chillura Martinoab,
Paolo Lo Meo*a and
Renato Notoa
aUniversità degli Studi di Palermo-Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche, V.le delle Scienze, Parco d’Orleans, pad. 17, 90128 Palermo, Italy. E-mail: paolo.lomeo@unipa.it
bCGA-ATeN Center – Università di Palermo, Via F. Marini, 14, 90128 Palermo, Italy
First published on 17th May 2016
Abstract
New pH-responsive nanosponges were obtained by reacting four different polyaminocyclodextrins with heptakis-(6-bromo)-(6-deoxy)-β-cyclodextrin. The materials obtained were characterized by various techniques (FT-IR, potentiometric titration, differential scanning calorimetry (DSC), porosimetry (BET), 13C{1H} CP-MAS NMR). Their adsorption abilities at different pH values were verified towards a suitable set of model guests, and seem mainly controlled by electrostatic interactions, as a function of the protonation/charge status of the polymer matrix. By contrast, data positively point out a lesser importance assumed by the induced-fit effect, important in affecting the formation of host–guest complexes in solution. The frequency-switched Lee-Goldburg (FSLG) heteronuclear correlation solid-state NMR technique was exploited in order to assess the possible location of the guests within the polymer matrix.
Introduction
Nanosponges (NSs) are a class of hyper-reticulated polymeric material,1 which have attracted increasing interest during the past years, due to their adsorption or controlled release abilities towards various organic molecules, such as drugs,1,2 conservation agents3 and pollutants.4 These materials can be obtained by polymerizing or co-polymerizing macrocyclic species able to act as supramolecular hosts. Great attention has been devoted in particular to cyclodextrin-based NSs, which are usually obtained by treating native β-cyclodextrin (βCD) with double electrophiles such as epichlorohydrin,5 carbonic acid derivatives6 or bis-isocyanates.7 The reaction, of course, exploits the nucleophilic reactivity of the hydroxyl groups (both primary and secondary) on the βCD scaffold, whereas the electrophile residues function as linkers between the βCD subunits. Very recently, we have succeeded in exploiting a different approach,8 i.e. a CuAAC reaction9 between heptakis-(6-azido)-(6-deoxy)-βCD and a tetrakis-propargyloxy-calix[4]arene derivative. This procedure affords mixed co-polymers, having different average compositions depending on the combination ratio of the reactants. The potential use of these materials takes advantage of the somehow complementary binding abilities possessed by the two diverse macrocyclic hosts, and we verified that their adsorption abilities largely vary as a function of their actual composition.An interesting aspect related to the latter issue is the potential tunability of the co-polymers obtained, which can be hardly achieved for NSs based on βCD only. Sensitivity to external stimuli is an appealing goal in materials science.10 In particular, systems able to change their properties in response to pH variations constitute an expanding area of research.11 Just to cite a few examples, pH-tunable nanochannels for selective transmembrane ion transport have been built by means of polyelectrolyte brushes12 or functionalized carbon nanotubes.13 Molecular shuttles triggered by pH variations can mimic “molecular muscles”.14 Reversible pH-dependent emulsifying action has been reported for chitosan.15 Mesoporous silica containers equipped with supramolecular nanovalves have been used for drug delivery into acid generating cells.16 In this context, the synthesis of pH-responsive NSs may constitute a very interesting task, in particular for the construction of drug carrier/delivery systems or pollutant removal devices.
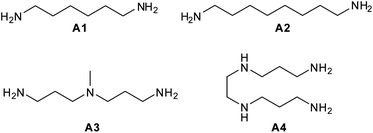
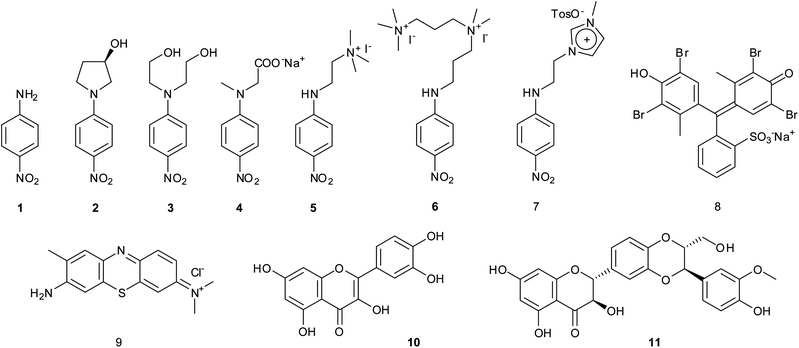
In the present work we report on the preparation, characterization, and sequestering abilities of a new class of NS materials, namely aminocyclodextrin nanosponges (ACN), obtained joining (6-deoxy)-βCD subunits by means of different aliphatic polyamine linkers. In particular, we chose two diamines, namely 1,6-diaminohexane (A1) and 1,8-diamino-octane (A2), and two polyamines, namely bis-N,N-(3-amino-propyl)-methyl-amine (A3) and bis-N,N′-(3-aminopropyl)-1,2-diamino-ethane (A4) (Fig. 1) as the linkers. These molecules differ in their length and hydrophobic character as well. The materials obtained ACN1–ACN4 were characterized by means of different complementary techniques, namely FT-IR, potentiometric titration, Differential Scanning Calorimetry (DSC), porosimetry (BET), 13C{1H} CP-MAS and Frequency-Switched Lee-Goldburg (FSLG) 13C–1H heterocorrelation solid-state NMR. Their adsorption abilities were evaluated towards a set of suitable model guests (1–11, Fig. 2), in buffered systems at different pH values (namely, 1.0, 4.4, 6.7 and 10.5).
The guests were chosen in such a way to show significant variations in molecular properties such as the hydrophobic/hydrophilic character, the charge status and the molecular volume. In particular, p-nitroaniline derivatives 1–7 have been selected because similar molecules have been largely used to assess the microscopic interactions occurring in the formation of supramolecular complexes with βCD.17,18 Guests 8–11 possess large and quite rigid structures with diverse features. Bromochresol Green 8 and Toluidine Blue and 9 are an anionic and a cationic dye respectively, with largely delocalized charges. Quercetin19 8 and Silibinin20 9 are two nutraceuticals, the bioactivity and potential anti-cancer activity of which has been extensively assessed. Noticeably, guests 4 and 9–11 change their protonation status, passing from a neutral to an anionic form, on increasing the pH.
Results and discussion
Synthesis of materials ACN1–ACN4
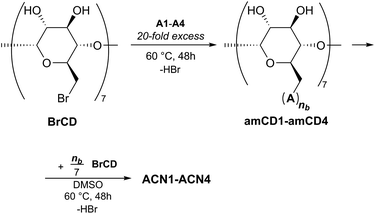
In order to accomplish the syntheses of our ACNs, we exploited a different approach with respect to previous literature. Because of the intrinsically nucleophilic nature of the polyamine linkers, we reasoned that the βCD residues could logically constitute the electrophilic synthons of a trivial nucleophilic displacement reaction. The easily accessible heptakis-(6-deoxy)-(6-bromo)-βCD (BrCD)21 appeared a suitable candidate as the starting material. It is worth mentioning here that the heptakis-(6-halo)-βCDs are able to react solvent-free with a strong excess of amines A3 and A4 to give the corresponding polyaminocyclodextrin (amCD) derivatives.22 Due to the occurrence of polysubstitution side-processes, the reaction actually affords mixtures of products bearing a different number of polyamine branches. However, these products can be fully characterized, and have been suitably used as capping auxiliaries for the synthesis of catalytically active silver nanocomposites.22,23In a first series of experiments, we tried to react directly BrCD and amines A1–A4 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mol/mol ratio. The carefully blended solvent-free semi-fluid mixture of the reactants was kept at 60 °C for 48 h, in order to ensure that the nucleophilic displacement reaction came to completion. Unfortunately, in this way we were able to obtain only intractable, partly water-soluble, gummy slurries.
7 mol/mol ratio. The carefully blended solvent-free semi-fluid mixture of the reactants was kept at 60 °C for 48 h, in order to ensure that the nucleophilic displacement reaction came to completion. Unfortunately, in this way we were able to obtain only intractable, partly water-soluble, gummy slurries.
Then, we tried a two-step approach (Fig. 3), i.e. we first synthetized four polyaminocyclodextrin derivatives amCD1–amCD4, by reacting BrCD with amines A1–A4;22 then the intermediate products obtained were again reacted with BrCD. More in detail, to accomplish the first step a 20-fold excess of the proper polyamine was used, which served both as reactant and as the reaction solvent. High-resolution ESI-MS and potentiometric titration characterization (see Experimental for details) enabled us to verify the presence of the expected products and the average number (nb) of polyamine branches attached to the βCD scaffold. In particular, we found nb values as large as 5.5 for amCD1, 5.8 for amCD3 and 4.5 for amCD4; the latter two values are in perfect agreement with literature reports.22 On the other hand, an anomalous 7.3 value was found for amCD2, indicating that the isolation and purification procedure used was not able to remove the last traces of unreacted diamine A2 from the product. The latter finding can be reasonably attributed to the formation of a very stable host–guest complex between the highly hydrophobic A2 molecule and the CD derivative. However, we reasoned that this fact would not have been relevant for the proceeding of the synthetic project. Indeed, in the second step we simply allowed the obtained amCD1–amCD4 products to react with BrCD in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nb mol/mol combination ratio (Rt). Noticeably, this choice corresponds to a 1
nb mol/mol combination ratio (Rt). Noticeably, this choice corresponds to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equivalent ratio between the two reactants. The reaction was performed in the presence of a very small amount of DMSO (ca. 400 μL per g of reactants mixture) just to ensure the most intimate blending of the reactants. This time we were actually able to obtain the desired polymers as brown amorphous materials which, after repeated washings with methanol and diethyl ether and subsequent drying, could be easily grinded (<150 μm) to afford the final products as yellowish powders, in very high yields (>95%, see later).
1 equivalent ratio between the two reactants. The reaction was performed in the presence of a very small amount of DMSO (ca. 400 μL per g of reactants mixture) just to ensure the most intimate blending of the reactants. This time we were actually able to obtain the desired polymers as brown amorphous materials which, after repeated washings with methanol and diethyl ether and subsequent drying, could be easily grinded (<150 μm) to afford the final products as yellowish powders, in very high yields (>95%, see later).
Characterization of materials ACN1–ACN4
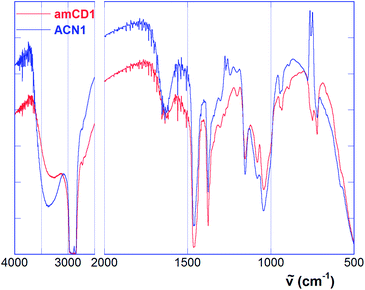
FT-IR spectra of amCD1 and the material ACN1 are depicted in Fig. 4 as representative examples. As we can easily see, both spectra show the same main features. In particular, we can notice a system of three peaks at 1155, 1084 and 1041 cm−1 (together with a minor signal at 946 cm−1) constituting the typical fingerprint of the CD scaffold, and the N–H wagging band at 722 cm−1.23 Spectra also show the large and intense O–H stretching band in the region over 3200 cm−1, which conceals possible N–H stretching bands. Finally, the presence of residual traces of water is accounted for by the jagged H–O–H bending band centered at ca. 1627 cm−1.
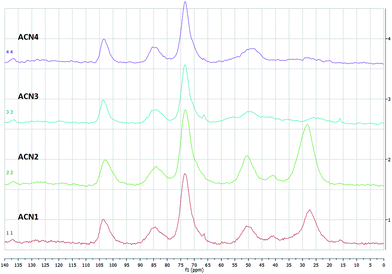
Solid state 13C{1H} CP-MAS24 NMR spectra of our materials are synoptically illustrated in Fig. 5. All spectra show a system of three signals accounting for the CD scaffold. In particular, we notice a signal centred at ca. 103 which can be attributed to the anomeric C(1), and two partly overlapped signals at ca. 84 and 73 ppm, the first one attributable to the C(4), the second one cumulatively to the C(2), C(3) and C(5) carbon atoms. Moreover, the spectra of materials ACN1 and ACN2 show two broad signals centred at ca. 50 and 27 ppm, together with a further minor signal at ca. 41 ppm. The signals at ca. 50 and 41 ppm can be reasonably attributed to C atoms directly bound to a nitrogen atom, i.e. the C(6) of the CD scaffold and the end-chain C atoms of the diamine linkers A1 or A2. The signal at ca. 27 ppm, in turn, can be attributed to the other C atoms of the linker chains. On the other hand, in the spectra of ACN3 and ACN4 no signal at 41 ppm is any longer visible, whereas the signal at 27 ppm appears very low in intensity. The latter finding is consistent with the fact that in the relevant linker chains only a minority of carbon atoms is not bound to a nitrogen atom.
Along with a qualitative confirmation of the simultaneous presence of the βCD scaffold and the linker structure, the 13C{1H} CP-MAS NMR technique may afford even semi-quantitative information. As a matter of fact, under the hypothesis that cross-polarization effects work homogeneously for all the carbon atoms present in the sample, in principle integration analysis of the signals might be performed.8,25 However, this cannot be assumed a priori, and must be somehow validated. The peaks integrals for the four spectra, obtained by taking the signal of the anomeric C(1) as the reference standard, are collected in Table 1.
| Attribution | ppm | Signal integration | |||
|---|---|---|---|---|---|
| ACN1 | ACN2 | ACN3 | ACN4 | ||
| a C of the linker chain bound to a nitrogen atom.b C of a linker chain not bound to a nitrogen atom. | |||||
| CCD(1) | 103 | 1 | 1 | 1 | 1 |
| CCD(4) + CCD(2,3,5) | 84, 73 | 4.14 | 4.40 | 4.00 | 4.17 |
| CCD(6) + Clinker,Na | 50, 41 | 1.30 | 1.63 | 2.09 | 2.00 |
| Clinker,Cb | 27 | 2.34 | 3.85 | 0.71 | 0.36 |
As long as the signals relevant to the CD scaffold are concerned, agreement between the expected integral values and the ones found seems satisfactory. Considering the slightly overlapped signals at 84 and 73 ppm cumulatively, in three cases deviation from the expected value of 4.0 is less than 5%, and thus can be considered fully acceptable within the experimental errors. On the other hand, a 10% deviation is found for ACN2. Unfortunately, attempt to extend this analysis to the signals of the linkers afforded more problematic results. For materials ACN1 and ACN2 constituted with the diamine linkers A1 and A2 respectively, if each glucose moiety of a given βCD subunit would bind a different linker chain, then values should be expected for the integration of the signal at 27 ppm as large as 2.0 and 3.0 respectively. This corresponds to the limit hypothesis that no polysubstitution side-reactions would have occurred in the formation of the polymer network. Experimental values are larger than these upper limits, indicating that for the relevant –CH2– groups cross-polarization effects work much more effectively as compared to the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) CH– groups of the βCD. This is the obvious consequence of having a larger number of hydrogens bound to the same C atom.26 For materials ACN3 and ACN4 a reliable integration of the signal at 27 ppm is made difficult by its aforementioned low intensity. On the other hand, it is noteworthy that cumulative integration of the signals centered at 50 and 41 ppm renders values that are lower than the relevant theoretical upper limits (i.e. 2 for ACN1 and ACN2, 3.5 for ACN3, and 4 for ACN4). This finding can be justified by the unfavorable outcome of proximate quadrupolar nuclei such as 14N to the transmission of cross-polarization effects,27 which in fact compensates the enhancement observed for the other –CH2– groups discussed previously. Then, by simple algebraic passages, relevant data enable us to estimate an average number of polyamine linkers per βCD subunit (nl) as large as 2.1 ± 0.2 for ACN1, 4.4 ± 0.5 for ACN2, 3.0 ± 0.3 for ACN3 and 2.3 ± 0.2 for ACN4.
CH– groups of the βCD. This is the obvious consequence of having a larger number of hydrogens bound to the same C atom.26 For materials ACN3 and ACN4 a reliable integration of the signal at 27 ppm is made difficult by its aforementioned low intensity. On the other hand, it is noteworthy that cumulative integration of the signals centered at 50 and 41 ppm renders values that are lower than the relevant theoretical upper limits (i.e. 2 for ACN1 and ACN2, 3.5 for ACN3, and 4 for ACN4). This finding can be justified by the unfavorable outcome of proximate quadrupolar nuclei such as 14N to the transmission of cross-polarization effects,27 which in fact compensates the enhancement observed for the other –CH2– groups discussed previously. Then, by simple algebraic passages, relevant data enable us to estimate an average number of polyamine linkers per βCD subunit (nl) as large as 2.1 ± 0.2 for ACN1, 4.4 ± 0.5 for ACN2, 3.0 ± 0.3 for ACN3 and 2.3 ± 0.2 for ACN4.
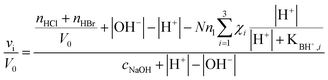 | (1) |
| ACN1 | ACN2 | ACN3 | ACN4 | |
|---|---|---|---|---|
| nl | 2.2 ± 0.2 | 2.8 ± 0.1 | 2.6 ± 0.2 | 2.1 ± 0.2 |
| x | 2.6 ± 0.2 | 3.1 ± 0.2 | 3.4 ± 0.3 | 3.4 ± 0.5 |
| χ1 | 0.14 ± 0.01 | 0.16 ± 0.01 | 0.27 ± 0.02 | 0.25 ± 0.01 |
| pKBH+,1 | 5.7 ± 0.4 | 6.1 ± 0.3 | 5.6 ± 0.3 | 5.1 ± 0.2 |
| χ2 | 0.43 ± 0.03 | 0.25 ± 0.02 | 0.36 ± 0.04 | 0.27 ± 0.03 |
| pKBH+,2 | 7.6 ± 0.3 | 7.5 ± 0.3 | 7.5 ± 0.2 | 7.5 ± 0.3 |
| χ3 | 0.43 ± 0.03 | 0.59 ± 0.03 | 0.37 ± 0.04 | 0.48 ± 0.04 |
| pKBH+,3 | 9.4 ± 0.5 | 8.9 ± 0.3 | 9.5 ± 0.2 | 9.5 ± 0.3 |
| 〈FW〉 | 1480 ± 80 | 1660 ± 30 | 1660 ± 40 | 1650 ± 70 |
| Rt | 1.27 | 0.97 | 1.21 | 1.46 |
| Rr | 0.67 ± 0.11 | 0.62 ± 0.05 | 0.81 ± 0.08 | 0.88 ± 0.16 |
We can immediately notice that in three cases nl values obtained from titration curves are consistent with those estimated by NMR. On the other hand, for material ACN2 the disagreement observed seems to parallel the concomitant overestimation of the cumulative integral for the signals at 84 and 73 ppm mentioned previously. Of course, because each linker binds two different βCD subunits, the actual average number of polyamine chains linked to a single βCD is 2nl. By means of trivial algebraic passages, from data reported in Table 2 we can easily calculate an average formula weight (〈FW〉) and a real amCD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BrCD mol/mol combination ratio (Rr) for each material. The latter one, in turn, can be compared with the mol/mol ratio (Rt) used in performing the reaction.
BrCD mol/mol combination ratio (Rr) for each material. The latter one, in turn, can be compared with the mol/mol ratio (Rt) used in performing the reaction.
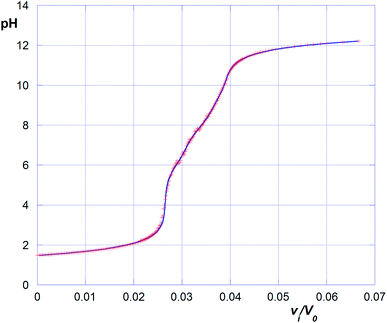
Titration experiments confirm the presence of HBr bound to the polymer. Moreover, for all the materials we can notice that Rr values are always much lower than the corresponding Rt values. This implies that a significant fraction of the amCD used in the synthesis does not react with the BrCD. Consequently, it does not participate in constituting the polymer network and is lost during work-up (it can be recovered from the washing liquors). The amount of unreacted amCD ranges between 33% (amCD3) and 47% (amCD1). The particularly low Rr value found for material ACN2 is justified by the fact that the relevant amCD2 reactant carries a certain amount of unbound diamine A2, which in turn contributes in the construction of the resulting network. Based on the amount of BrCD used for the synthesis, calculated reaction yields resulted almost quantitative, but for the trivial losses involved in the isolation procedures. It is also worth noting that Rr values seem to increase on increasing the number of nitrogen atoms per polyamine linker unit. These observations can be rationalized assuming that a single amCD unit substitutes more than one bromine atom of the same BrCD unit. It can be reasonably admitted that the hyper-reticulated nature itself of the resulting polymer network restricts the actual number of BrCD units that a given amCD can spatially approach to react with. This, of course, favors the occurrence of polysubstitution side-reactions. The observed trend of Rr values implies that an increase in the overall number of N atoms on the same amCD unit improves its ability to approach different BrCD units at the same time.
It is worth mentioning here that the titration of polyelectrolytes has been object of various studies and has been approached in different ways.28–30 For instance, in the case of polycarboxylic acids such as acrilic29 or crotonic30 acid polymers, bearing formally equivalent ionizable groups, data relevant to the titration with a strong base have been treated according of the following eqn (2):
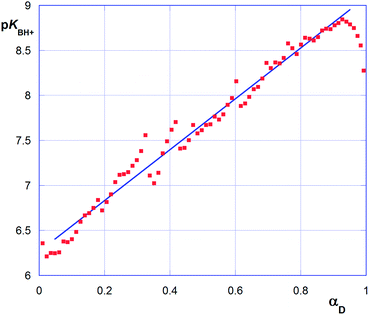
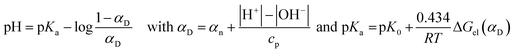 | (2) |
The continuous increase in apparent pKBH+ values, during the titration experiment, accounts for an increasing difficulty in extracting protons from the polymeric matrix, as the overall positive charge of the polymer decreases. From data plotted in Fig. 7 we can observe that pKBH+ values generally spun over a quite large range (ca. 3 log units), as compared for instance with the case of acrylic acid polymers or co-polymers reported in literature. The latter observation can be easily justified considering the different substitution of the N amine atoms (which can be tertiary, secondary, or even primary) present in the material. Noticeably, the estimated pKBH+,0 values (ca. 7.5–8.0) appear on average larger than the typical pKBH+ values for aliphatic amines. Even keeping into account the aforementioned large variation range, we can conclude that the amine groups present in the polymer matrix appear on average weaker bases than free aliphatic amines. This behavior can be reasonably attributed to the less extensive solvation occurring within the matrix, which destabilizes the protonated ammonium form as compared with free species in solution.
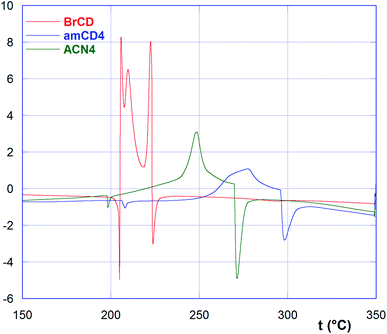
As we can easily see, BrCD shows a very peculiar trend, with three exothermic peaks centered at 206, 210 and 223 °C, comprised between two endothermic ones at 205 and 224 °C. On the other hand, free amCDs and ACNs show very similarly shaped curves showing first a tiny endothermic peak at ca. 200 °C, followed by a large exothermic peak between 240 and 280 °C, and finally a third significant endothermic peak between 250 and 310 °C. Data are summarized in Table 3.
According to literature,31 BrCD decomposes on melting at ca. 220–223 °C. So, the exothermic peaks in its peculiar DSC curve may be interpreted as due to the occurrence of some amorphous–christalline transitions just before thermal decomposition at 224 °C. No literature data are available on the thermal behavior of AmCDs. In general, data indicate that, with the exception of material ACN2, the polymers are less stable as compared to the relevant AmCDs. The first tiny endothermic peak in the curves might be attributed to a first melting process, although loss of HBr cannot be a priori excluded. Then, the broad exothermic peak could be ascribable to an amorphous–crystalline transition, ultimately followed by partial thermal degradation. Noticeably, a somehow similar behavior has been observed for composites in which an endothermic melting is followed by an exothermic crystallization and hence by the melting of the new structure.32 As the mobility of portion of the molecules is increased in the molten state, an imperfect crystallization could occur, as observed in particular for bio-polyamides. Higher melting point can account for the occurrence of extensive hydrogen bonding, which affects the order of microchains in the molten state.33
| Material | pH | Guest | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| a All data given with a ±3% indetermination. | ||||||||||||
| ACN1 | 1.0 | 94 | 22 | 61 | 75 | 6 | 30 | 20 | 78 | 2 | 94 | 88 |
| 4.4 | 89 | 30 | 55 | 71 | 0 | 21 | 19 | 81 | 4 | 92 | 93 | |
| 6.7 | 85 | 45 | 42 | 58 | 19 | 37 | 39 | 91 | 7 | 87 | 91 | |
| 10.5 | 81 | 51 | 33 | 42 | 30 | 50 | 51 | 35 | 45 | 1 | 18 | |
| ACN2 | 1.0 | 88 | 12 | 5 | 55 | 0 | 5 | 9 | 93 | 4 | 99 | 94 |
| 4.4 | 83 | 18 | 9 | 53 | 0 | 8 | 8 | 94 | 6 | 99 | 99 | |
| 6.7 | 76 | 26 | 13 | 38 | 6 | 15 | 17 | 98 | 5 | 98 | 97 | |
| 10.5 | 70 | 34 | 29 | 33 | 20 | 29 | 34 | 37 | 48 | 12 | 8 | |
| ACN3 | 1.0 | 94 | 15 | 6 | 61 | 0 | 9 | 27 | 99 | 4 | 98 | 91 |
| 4.4 | 68 | 21 | 13 | 44 | 2 | 10 | 4 | 98 | 5 | 97 | 94 | |
| 6.7 | 56 | 30 | 25 | 25 | 8 | 16 | 18 | 77 | 8 | 83 | 89 | |
| 10.5 | 48 | 23 | 20 | 38 | 22 | 37 | 38 | 52 | 36 | 8 | 30 | |
| ACN4 | 1.0 | 80 | 9 | 5 | 55 | 0 | 9 | 16 | 56 | 4 | 96 | 93 |
| 4.4 | 65 | 22 | 14 | 26 | 0 | 7 | 7 | 98 | 4 | 96 | 92 | |
| 6.7 | 52 | 25 | 38 | 18 | 8 | 15 | 18 | 53 | 12 | 97 | 87 | |
| 10.5 | 31 | 39 | 39 | 45 | 29 | 44 | 39 | 37 | 22 | 4 | 29 | |
The sequestration abilities of the polymers show very large variations depending on the nature of guest and on the pH. Comparison between homologous data for the different materials shows that possible cross-correlations are only fair, at the best. On a statistical basis, ACN1 shows on average slightly better adsorption abilities than the other materials. Comparisons by pH show fair cross-correlations between data at pH 1.0, 4.4 and 6.7, whereas data at pH 10.5 are essentially uncorrelated with the other ones. Considering the protonation status of the materials at the different pH values, these results indicate that significant modifications in the binding abilities and selectivities occur on changing the overall positive charge on the polymer lattice. These observations confirm the expected pH-responsivity of the materials, and are in agreement with the behavior observed for amino-cyclodextrin derivatives in solution.18 Of course, the response of the materials to pH variations changes depending on the particular guest considered. For the cationic guests 5, 6, 7 and 9, for instance, the protonated materials are by far worse adsorbents, whereas the opposite is observed with anionic or ionizable guests 2, 8, 10 and 11. Indeed, data reported in Table 4 may be analyzed in detail also keeping into account the peculiar features of the different substrates.
The neutral parent p-nitroaniline 1 is sequestered quite efficiently in particular by the diamine derived materials ACN1 and ACN2, whose aliphatic linkers are less hydrophilic, whereas less effective adsorption is observed by materials ACN3 and ACN4 bearing more hydrophilic polyamine linkers. It is noteworthy that 1 is more favorably adsorbed by the fully protonated materials at pH 1.0, in striking disagreement with the behavior observed in solution with some mono-(6-amino)-(6-deoxy)-βCD derivatives.18 In the latter case, a decreased stability for the host–guest complex in solution at acidic pH values had been explained as the consequence of entropically unfavorable stiffening effects, occurring as the positively charged host interacts with the polarized guest molecule. We may reason that a similar effect cannot work properly in the already stiff hyper-reticulated structure of the polymer; thus, inclusion is ultimately favored by the occurrence of stronger polar interactions.
On passing to the other p-nitroaniline derivatives 2–7, we noticed that these guests are always less favorably adsorbed than the parent 1 (with the only exception of material ACN4 at pH 10.5), irrespective of the size and features of the ancillary chain present on the molecule. As long as neutral guests 2 and 3 are concerned, their adsorption is now disfavored by pH decrease (with the only exception of 3 with ACN1), coherently with the behavior of aminocyclodextrins in solution. Moreover, as we mentioned previously, electrostatic interactions also play an important role. For the carboxylic acid derivative 4 the best adsorptions occur at pH 1.0, and regularly decrease with ACN1 and ACN2, whereas pass through a minimum with ACN3 and ACN4. Probably, in the latter two cases the accommodation of the guest into the βCD cavities is disfavored by the occurrence of effective hydrogen bonding and Coulomb interactions between the carboxylate group and the ammonium groups of the polyamine linkers occurring at intermediate pH values. Because these interactions are lost as the polymer becomes completely deprotonated, then sequestration abilities pass through a minimum. The adsorptions of the alkylammonium cations 5 and 6 regularly decrease on decreasing the pH with all the materials. The same trend is found with the imidazolium cation 7 in the presence of ACN1 and ACN2, whereas non-monotonic trends are found with ACN3 and ACN4. Keeping into account that cation 7 is accompanied by tosylate as the counter-anion, this anomalous behavior can be explained admitting the occurrence of a concomitant strong interaction between the aromatic anion and the cationic polymer occurring under strongly acidic conditions.
Considering now large guests 8–11, we noticed that increased molecular size does not necessarily exert an unfavorable effect on sequestration. Cationic Toluidine Blue 9, indeed, is fairly adsorbed only under alkaline conditions, and always less than p-nitroaniline 1. However, if we consider Bromochresol Green 8, Quercetine 10 and Silibinine 11, under the various conditions used, in 35 cases out of 48 these large molecules are sequestered as well as or even much more efficiently than the smallest p-nitroaniline 1. More in detail, 8 (which turns from a neutral to a largely delocalized anionic form at around pH 4.8) shows bell-shaped trends. Therefore, the best adsorption conditions are achieved at intermediate pH values, i.e. as the anionic form of the guest interacts with the polymer in its partly cationic form. This behavior is particularly apparent with material ACN4. Therefore, possible interaction with the polyamine linkers this time may plays a favorable role, in contrast with the behavior observed for anion 4. Nutraceuticals 10 and 11 are almost quantitatively sequestered by all the materials under acidic or nearly neutral pH. By contrast, adsorptions fall dramatically down under alkaline conditions, due to the deprotonation of the molecule (which becomes a much more hydrophilic anion) and the concomitant loss of charge for the polymer. It is also worth noting that, unlike p-nitroanilines 1–7, the material ACN1 bearing the shortest linker chains does not show towards these large guests the best sequestration abilities. Indeed, as compared with the other three materials, ACN1 works neatly better only in six cases out of 48.
A general consideration of these data indicates that adsorption/sequestration abilities of our materials can hardly be compared with the binding abilities of free cyclodextrins in solution. It is worth recalling here that the latter ones are finely regulated by the well-known “induced-fit effect”.17,36 The conformational rearrangement of the fairly flexible cyclodextrin scaffold upon the structure of the guest, indeed, optimizes the interplay between the various possible interactions involved at a microscopic level. However, this mechanism fails for our materials as we mentioned previously, because of their intrinsically stiff and relatively poorly solvated structure. Of course, adsorption (and binding in solution for the free species as well) must be strictly controlled by the different possible microscopic interactions involved (namely, electrostatic and van der Waals interactions, hydrogen bonding, etc.) and by solvation/desolvation effects. So, the question arises of which is the actual location of the adsorbed guest within the material lattice. For instance, p-nitroanilines are well known to be included by free cyclodextrins in such a way that the aromatic moiety directs its nitro-group towards the primary host rim. On the other hand, very large guests cannot be comfortably accommodated into a single βCD unit; in several cases 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry complexes may occur. Therefore, the possibility that the guest might reside in the βCD subunits and/or in the nanochannels formed within the polymer lattice as well, must be taken into account.
2 stoichiometry complexes may occur. Therefore, the possibility that the guest might reside in the βCD subunits and/or in the nanochannels formed within the polymer lattice as well, must be taken into account.
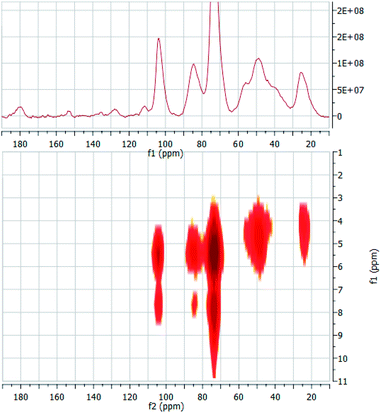
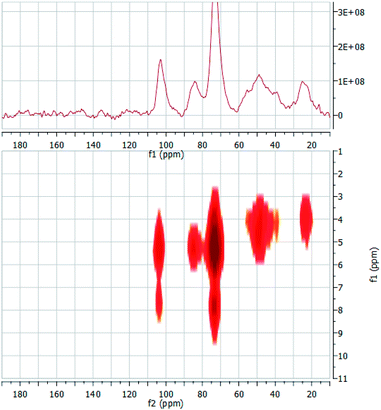
In order to gain this piece of information, we prepared two composites by adsorbing guests 2 and 10 into material ACN3. The composites were easily obtained (see Experimental) by equilibrating the polymer with a suitable amount of the guests for a sufficiently long time. Loadings were determined by extracting the included guest, and resulted as large as 11% w/w for the 2·ACN3 composite, and 5% w/w for the 10·ACN3 one. On the grounds of trivial stoichiometric calculations, it can be easily shown that loading for 2·ACN3 corresponds to an almost complete occupancy of the cyclodextrin subunities present in the material, whereas for 10·ACN3 occupancy is ca. 30%. The composites obtained were subsequently used to perform solid-state 2D (1H–13C) heteronuclear dipolar correlation NMR experiments, using the frequency-switched Lee-Goldburg homonuclear decoupling sequence (FSLG). This technique enables to detect through-space H–C cross-correlations, and therefore it affords information somehow comparable to the well-known ROESY spectra in solution. The 1D CP-MAS and a low-magnification 2D FSLG spectrum of the composite 2·ACN3 are shown synoptically in Fig. 9; the analogous spectra for the composite 10·ACN3 are depicted in Fig. 10.
The 1D spectrum of the 2·ACN3 composite clearly shows the presence of low intensity peaks at 111.5, 128.1, 135.7 and 153.5 ppm relevant to aromatic carbon atoms, which account for the presence of 2 embedded in the polymer matrix (a further signal at ca. 180 ppm is due to acetate ion, deriving from the aqueous buffer used in the preparation of the composite). Accordingly, comparison with the spectrum of ACN3 alone shows a significant enhancement of the signals relevant to the high-field aliphatic carbons (ca. 50 and 27 ppm), which can be attributed to the contribution of the aliphatic C atoms of the pyrrolidine moiety of the guest. At the same time, we can notice a slight enlargement of the signal relevant to the anomeric C(1) of theCD scaffold. On passing to the 2D correlation spectrum, we have preliminarily to outline that all 1H resonances undergo a significant downfield shift with respect to solution. This effect is particularly apparent for aliphatic hydrogen atoms, in particular for those of the linkers, which now resonate below 3.5 ppm. In the 2D spectrum we can first notice three cross-peaks at δH = 7.82 ppm and δC = 73.7 ppm, at δH = 7.75 ppm and δC = 84.7 and at δH = 7.68 ppm and δC = 104.2. These signals undoubtedly account for the interaction, and therefore the spatial proximity, between the aromatic hydrogen atoms of the guest and the CD carbons. This finding, together with the simultaneous lack of any intense cross-peak between the same hydrogens and the carbon atoms of the linker units, provides unambiguous evidence that the aromatic moiety of 2 is stably included into the βCD subunits, rather than reside in the nanochannels of the polymer. It is worth noting, indeed, that the first cross-peak is by far more intense, in agreement with the fact that the C(3) and C(5) βCD atoms result closer in the space to the guest, with respect to the C(1) and C(4) atoms. The 2D spectrum shows also a cross-peak at δH = 4.59 ppm and δC = 49.3 and a weaker one at δH = 4.25 ppm and δC = 24.2 ppm, which likely account for the interaction between the linker carbons and the outer hydrogens (H(2), H(4) and H(6,6′)) of the CD subunits. Finally, high-magnification 2D spectra (not shown for brevity) enables to detect very weak cross-peaks at δH = 5.0 ppm and δC = 112.2 ppm and at δH = 4.9 ppm and δC = 127.5 ppm, which can be tentatively attributed to the interaction between the aromatic C atoms of the guest and the inner-cavity H(3) of the CD.
On passing to the composite 10·ACN3, once again we easily verify that the signals relevant to the guest can be easily recognized in the 1D spectrum, despite the lower loading. In particular, we can notice a system of tiny signals between 113 and 126 ppm, relevant to carbons of the pyrocatechine-like moiety. Other signals are constituted by a second cluster between 135 and 145 ppm, and three signals at ca. 158, 162 and 176 ppm, together with a shoulder at ca. 95 ppm. The 2D spectrum shows two correlation peaks at δH = 8.1 ppm and δC = 73.6 ppm and at δH = 7.6 ppm and δC = 103.6 ppm, which suggest a spatial proximity between the pirocatechine-like moiety of the guest and the βCD cavity. This seems confirmed by a weak cross-correlation peak, again apparent only at large magnification, at δH = 5.3 ppm and δC = 116.9 ppm. Therefore, these findings provide convincing evidence that the guest is only partly included in the βCD subunits, whereas the chromone-like moiety fluctuates out of the cavity, in the nanochannels region. Noticeably, no interaction seems apparent from the 2D spectrum between this moiety and the linker carbons, indicating a possible mobility up to certain extent.
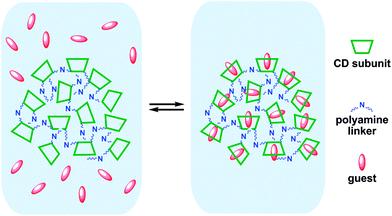
On the whole, these evidences seem to support the ideas exposed in discussing the sequestration data. Indeed, inclusion of p-nitroanilines suffers for the intrinsic rigidity of the βCD subunits, because these guests specifically interact with them (a possible pictorial representation of the absorption equilibrium is shown in Fig. 11). On the other hand, in the case of large guests, only part of the molecule can get into the host residues, whereas the largest part resides in the nanochannels, interacting only shallowly with the linker chains.
Experimental
Materials and instrumentation
All commercial materials needed were used as purchased (Aldrich, Fluka) with no further purification. The non-commercial heptakis-(6-deoxy)-(6-boromo)-βCD (BrCD) was prepared according to literature.21 UV-vis spectra were recorded on a Beckmann DU 800 spectrophotometer. FT-IR spectra (nujol) were recorded with an Agilent Technologies Cary 630 FT-IR spectrometer. 1H NMR spectra (DMSO-d6) of amCDs were recorded on a Bruker AS 300 MHz instrument; whereas solid state NMR spectra were acquired using a Bruker Avance II 400 MHz instrument, equipped with a 15 kHz rotating MAS probe. ESI-MS mass spectra were acquired in positive mode on an AGILENT Technologies 6540 UHD Accurate Mass Q-TOF LC-MS apparatus (1 kV nozzle voltage, 250 V fragmentor voltage). DSC measurements were performed on a DSC Q20 TA Instruments apparatus. For porosimetric determinations, N2 absorption–desorption isotherms were registered at 77 K using a Quantachrome Nova 2200 Multi-Station High Speed Gas Sorption Analyzer.Synthesis of polyaminocyclodextrins amCD1–amCD4
Polyaminocyclodextrins were prepared according to the procedure illustrated elsewhere.22 The BrCD (315 mg, 0.2 mmoles) was mixed with a 20-fold excess (28 mmoles) of the proper polyamine, namely 3.25 g of A1, 4.04 g of A2, 4.75 mL (4.08 g) of A3 or 5.15 mL (4.90 g) of A4. The mixture was kept in an oil bath at 60 °C under inert atmosphere and stirring for 48 h. Then, 20 mL of methanol were added to the reaction crude, and the resulting solution was added dropwise under vigorous stirring to 250 mL of cold diethyl ether. The system was allowed to settle for a few hours and then decanted, giving a brownish slurry, which was dissolved in 10 mL of methanol and again dropped under stirring into 200 mL of diethyl ether, to afford a second amorphous solid. This dissolution–precipitation procedure was repeated other two or three times, until a pale yellow solid was obtained. The product was finally filtered off and dried under vacuum overnight at 50 °C.![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–CH2–); 2.78–3.06 (two overlapped br m, –NH–CH2– and H(6)CD); 3.31 (br d, H(2)CD); 3.42 (br s, H(4)CD); 3.61 (br t, H(3)CD); 3.66 (br s, H(5)CD); 4.83 (br s, H(1)CD; overlapped with br s, –OH, –NH–). 13C NMR δ: 25.82, 26.39, 26.88, 27.54, 28.10, 29.82 (–CH2–CH2–CH2–); 49.18, 49.59, 49.92 (–NH–CH2– and C(6)CD); 71.04 (C(5)CD); 72.86 (C(2)CD); 73.26 (C(3)CD); 83.41 (cluster, C(4)CD); 102.52 (C(1)CD). High-resolution ESI-MS (m/z): 1822.2168 [C84H168N14O28·H]+ (calcd 1822.2225); 1706.0933 [C78H152N12O28·H]+ (calcd 1706.0912); 1589.9717 C72H136N10O28·H+ (calcd 1589.9598); 922.6055 [C84H168N14O28·Na·H]2+ (calcd 922.6059); 911.6113 [C84H168N14O28·2H]2+ (calcd 911.6149); 893.5123 [C78H152N12O28·HBr·2H]2+ (calcd 864.5402); 864.5435 [C78H152N12O28·Na·H]2+ (calcd 864.5402); 853.5489 [C78H152 N12O28·2H]2+ (calcd 853.5492); 795.4899 [C72H136N10O28·2H]2+ (calcd 795.4836); 608.0762 [C84H168 N14O28·3H]3+ (calcd 608.0790); 569.3711 [C78H152N12O28·3H]3+ (calcd 569.3686).
N–CH2–); 2.78–3.06 (two overlapped br m, –NH–CH2– and H(6)CD); 3.31 (br d, H(2)CD); 3.42 (br s, H(4)CD); 3.61 (br t, H(3)CD); 3.66 (br s, H(5)CD); 4.83 (br s, H(1)CD; overlapped with br s, –OH, –NH–). 13C NMR δ: 25.82, 26.39, 26.88, 27.54, 28.10, 29.82 (–CH2–CH2–CH2–); 49.18, 49.59, 49.92 (–NH–CH2– and C(6)CD); 71.04 (C(5)CD); 72.86 (C(2)CD); 73.26 (C(3)CD); 83.41 (cluster, C(4)CD); 102.52 (C(1)CD). High-resolution ESI-MS (m/z): 1822.2168 [C84H168N14O28·H]+ (calcd 1822.2225); 1706.0933 [C78H152N12O28·H]+ (calcd 1706.0912); 1589.9717 C72H136N10O28·H+ (calcd 1589.9598); 922.6055 [C84H168N14O28·Na·H]2+ (calcd 922.6059); 911.6113 [C84H168N14O28·2H]2+ (calcd 911.6149); 893.5123 [C78H152N12O28·HBr·2H]2+ (calcd 864.5402); 864.5435 [C78H152N12O28·Na·H]2+ (calcd 864.5402); 853.5489 [C78H152 N12O28·2H]2+ (calcd 853.5492); 795.4899 [C72H136N10O28·2H]2+ (calcd 795.4836); 608.0762 [C84H168 N14O28·3H]3+ (calcd 608.0790); 569.3711 [C78H152N12O28·3H]3+ (calcd 569.3686).![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–CH3); 2.29, 2.32 (two overlapped br m,
N–CH3); 2.29, 2.32 (two overlapped br m, ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–CH2–); 2.50, 2.53 (two overlapped br m, –NH–CH2– and H(6)CD); 2.78 (br s, NH2–CH2–); 3.32 (br d, H(2)CD); 3.43 (br s, H(4)CD); 3.61 (br t, H(3)CD); 3.67 (br s, H(5)CD); 4.85 (br s, H(1)CD); 5.12 (br s, –OH, –NH–). 13C NMR δ: 27.31 (–CH2–CH2–CH2–); 38.47 (NH2–CH2–); 41.83 (
N–CH2–); 2.50, 2.53 (two overlapped br m, –NH–CH2– and H(6)CD); 2.78 (br s, NH2–CH2–); 3.32 (br d, H(2)CD); 3.43 (br s, H(4)CD); 3.61 (br t, H(3)CD); 3.67 (br s, H(5)CD); 4.85 (br s, H(1)CD); 5.12 (br s, –OH, –NH–). 13C NMR δ: 27.31 (–CH2–CH2–CH2–); 38.47 (NH2–CH2–); 41.83 (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–CH3); 48.22 (
N–CH3); 48.22 (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–CH2–); 48.70–49.70 (cluster, C(6)CD); 54.90, 55.76 (
N–CH2–); 48.70–49.70 (cluster, C(6)CD); 54.90, 55.76 (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–CH2–); 70.89 (C(5)CD); 72.73 (C(2)CD); 73.18 (C(3)CD); 82.64–83.49 (cluster, C(4)CD); 102.25–102.87 (cluster, C(1)CD). High-resolution ESI-MS (m/z): 1053.1682 [C91H189N21O28·HBr·2H]2+ (calcd 1053.1709); 1024.1977 [C91H189N21O28·Na·H]2+ (calcd 1024.1988); 1013.2068 [C91H189N21O28·2H]2+ (calcd 1013.2078); 980.5897 [C84H170N18O28·HBr·2H]2+ (calcd 980.5920); 951.6198 [C84H170 N18O28·Na·H]2+ (calcd 951.6198); 940.6287 [C84H170N18O28·2H]2+ (calcd 940.6289); 877.0572 [C77H151N15O28·H2O·2H]2+ (calcd 877.0550); 868.0492 [C77H151N15O28·2H]2+ (calcd 868.0495); 683.1345 [C91H189N21O28·Na·2H]3+ (calcd 608.0790); 675.8076 [C91H189N21O28·3H]3+ (calcd 675.8076); 627.4218 [C84H170N18O28·3H]3+ (calcd 627.4217); 579.0361 [C77H151N15O28·3H]3+ (calcd 579.0357) 507.1080 [C91H189N21O28·4H]4+ (calcd 507.1076); 470.8186 [C91H189N21O28·4H]4+ (calcd 470.8181).
N–CH2–); 70.89 (C(5)CD); 72.73 (C(2)CD); 73.18 (C(3)CD); 82.64–83.49 (cluster, C(4)CD); 102.25–102.87 (cluster, C(1)CD). High-resolution ESI-MS (m/z): 1053.1682 [C91H189N21O28·HBr·2H]2+ (calcd 1053.1709); 1024.1977 [C91H189N21O28·Na·H]2+ (calcd 1024.1988); 1013.2068 [C91H189N21O28·2H]2+ (calcd 1013.2078); 980.5897 [C84H170N18O28·HBr·2H]2+ (calcd 980.5920); 951.6198 [C84H170 N18O28·Na·H]2+ (calcd 951.6198); 940.6287 [C84H170N18O28·2H]2+ (calcd 940.6289); 877.0572 [C77H151N15O28·H2O·2H]2+ (calcd 877.0550); 868.0492 [C77H151N15O28·2H]2+ (calcd 868.0495); 683.1345 [C91H189N21O28·Na·2H]3+ (calcd 608.0790); 675.8076 [C91H189N21O28·3H]3+ (calcd 675.8076); 627.4218 [C84H170N18O28·3H]3+ (calcd 627.4217); 579.0361 [C77H151N15O28·3H]3+ (calcd 579.0357) 507.1080 [C91H189N21O28·4H]4+ (calcd 507.1076); 470.8186 [C91H189N21O28·4H]4+ (calcd 470.8181).![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) ), 2.60, 2.63 (two overlapped br m, –CH2–NH– and H(6)CD), 2.82 (m, –CH2–NH2 and H(6)CD), 3.35 (br s, H(2)CD); 3.50 (br s, H(4)CD), 3.66 (br s, H(3)CD), 3.76 (br s, H(5)CD), 4.91 (br s, H(1)CD), 4.95 (br s, –OH, –NH–); 13C NMR δ (ppm) 27.39, 28.32 (–CH2–CH2–CH2–), 38.48 (–CH2–NH2), 46.61, 47.36, 48.94 (–CH2–N
), 2.60, 2.63 (two overlapped br m, –CH2–NH– and H(6)CD), 2.82 (m, –CH2–NH2 and H(6)CD), 3.35 (br s, H(2)CD); 3.50 (br s, H(4)CD), 3.66 (br s, H(3)CD), 3.76 (br s, H(5)CD), 4.91 (br s, H(1)CD), 4.95 (br s, –OH, –NH–); 13C NMR δ (ppm) 27.39, 28.32 (–CH2–CH2–CH2–), 38.48 (–CH2–NH2), 46.61, 47.36, 48.94 (–CH2–N![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) ), 48.70–49.60 (cluster, C(6)CD), 70.36 (C(5)CD), 72.32 (C(2)CD), 72.80 (C(3)CD), 82.20–85.70 (cluster, C(4)CD), 102.45 (C(1)CD); high-resolution ESI-MS (m/z): 1114.8004 [C98H210N28O28·2H]2+ (calcd 1114.8007); 1038.6996 [C90H188N24O28·Na·H]2+ (calcd 1038.6995); 1027.7085 [C90H188N24O28·2H]2+ (calcd 1027.7085); 951.6077 [C82H166N20O28·Na·H]2+ (calcd 951.6073); 940.6166 [C82H166 N20O28·2H]2+ (calcd 940.6163); 853.5242 [C74H144N16O28·2H]2+ (calcd 853.5241); 743.5368 [C98H210 N28O28·3H]3+ (calcd 743.5363); 685.4752 [C90H188N24O28·3H]3+ (calcd 685.4748); 634.4719 [C82H166N20O28·Na·2H]3+ (calcd 634.4706); 627.4139 [C82H166N20O28·3H]3+ (calcd 627.4133): 569.3524 [C74H144N16O28·3H]3+ (calcd 569.3518); 557.9045 [C98H210N28O28·4H]4+ (calcd 557.9040); 514.3585 [C90H188 N24O28·4H]4+ (calcd 514.3579).
), 48.70–49.60 (cluster, C(6)CD), 70.36 (C(5)CD), 72.32 (C(2)CD), 72.80 (C(3)CD), 82.20–85.70 (cluster, C(4)CD), 102.45 (C(1)CD); high-resolution ESI-MS (m/z): 1114.8004 [C98H210N28O28·2H]2+ (calcd 1114.8007); 1038.6996 [C90H188N24O28·Na·H]2+ (calcd 1038.6995); 1027.7085 [C90H188N24O28·2H]2+ (calcd 1027.7085); 951.6077 [C82H166N20O28·Na·H]2+ (calcd 951.6073); 940.6166 [C82H166 N20O28·2H]2+ (calcd 940.6163); 853.5242 [C74H144N16O28·2H]2+ (calcd 853.5241); 743.5368 [C98H210 N28O28·3H]3+ (calcd 743.5363); 685.4752 [C90H188N24O28·3H]3+ (calcd 685.4748); 634.4719 [C82H166N20O28·Na·2H]3+ (calcd 634.4706); 627.4139 [C82H166N20O28·3H]3+ (calcd 627.4133): 569.3524 [C74H144N16O28·3H]3+ (calcd 569.3518); 557.9045 [C98H210N28O28·4H]4+ (calcd 557.9040); 514.3585 [C90H188 N24O28·4H]4+ (calcd 514.3579).Synthesis of materials ACN1–ACN4
ACN1: from 364.8 mg of amCD1 (181.9 μmoles). Yield 320.1 mg.
ACN2: from 330.8 mg of amCD2 (135.0 μmoles). Yield 354.9 mg.
ACN3: from 390.5 mg of amCD1 (172.0 μmoles). Yield 485.0 mg.
ACN4: from 495.8 mg of amCD2 (222.3 μmoles). Yield 535.1 mg.
Synthesis of guest 7
The [2-(p-nitrophenyl)-aminoethyl]-dimethylamine17a (1.09 g, 5.2 mmoles) and iodomethane (1.25 mL, 2.84 g, 20 mmoles) were dissolved in acetonitrile (10 mL) and solid K2CO3 (2.0 g) was added. The mixture was stirred for 24 h at room temperature. Then the solid crude was filtered off and crystallized from methanol. Yield 1.31 g (62%). m.f. 206–208 °C. IR (Nujol): 3424, 3193, 3173, 1596 cm−1. 1H-NMR (D2O) δ(ppm): 4.92 (s, 1H, –NH–), 5.19, 5.41 (2 t, 2H + 2H, –CH2CH2–, J = 6.4 Hz), 6.34 (s, 9H, –CH3), 8.30–9.68 (2d, 2H + 2H, ArH, J = 8.6 Hz). High-resolution ESI-MS (m/z): 224.1391 [C11H18N3O2]+ (calcd 224.1399).Synthesis of guest 8
To p-nitro-fluorobenzene (1.6 mL, 2.12 g, 15 mmol) dissolved in DMSO (15 mL) bis-N,N-(3-amino-propyl)-methyl-amine (7.5 mL, 6.1 g, 60 mmoles) was added and the mixture was kept under stirring at r.t. for 3 days. Then the mixture was poured into water (200 mL), acidified with conc. HCl (up to pH ca. 2) and washed with ethyl acetate. The aqueous layer was treated with NaOH (up to pH ca. 12) and repeatedly extracted with ethyl acetate. The collected organic extracts were dried on Na2SO4 and distilled under reduced pressure. The collected product was constituted by N1-(3-aminopropyl)-N1-methyl-N3-(p-nitro-phenyl)propane-1,3-diamine at a satisfactory degree of purity. Yield 2.76 g (82%). m.p. 52–54 °C. IR (nujol): 3541, 3239, 3173, 1605, 1466, 1298 cm−1; 1H-NMR (DMSO-d6) δ (ppm): 1.54 (m, 2H, –CH2–CH2–CH2–), 1.71 (m, 2H, –CH2–CH2–CH2–), 2.10 (s, 3H, –CH3), 2.30–2.43 (m, 4H,![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) NCH2–), 2.56 (t, 2H,
NCH2–), 2.56 (t, 2H, ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) NCH2–, J = 7.2 Hz), 3.15 (t, 2H,
NCH2–, J = 7.2 Hz), 3.15 (t, 2H, ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) NCH2–, J = 6.8 Hz), 6.54–7.96 (2d, 2H + 2H, ArH, J = 7.5 Hz). This intermediate product (1.33 g, 5 mmoles) was then dissolved in acetonitrile (10 mL); iodomethane (3 mL, 6.84 g, 48 mmoles) and solid K2CO3 (1.5 g) were added, and the mixture was stirred at r.t. for 24 h. The final crude product was filtered off and crystallized from methanol. Yield 2.25 g (78%). m.p. 207–210 °C. IR (nujol): ν 3421, 3238, 1594 cm−1. 1H-NMR (DMSO-d6) δ (ppm): 1.94–2.04 (m, 4H, 2 –CH2–CH2–CH2–), 3.08 (s, 9H, (CH3)3)N+–, 3.26 (br t, 2H –CH2–N+
NCH2–, J = 6.8 Hz), 6.54–7.96 (2d, 2H + 2H, ArH, J = 7.5 Hz). This intermediate product (1.33 g, 5 mmoles) was then dissolved in acetonitrile (10 mL); iodomethane (3 mL, 6.84 g, 48 mmoles) and solid K2CO3 (1.5 g) were added, and the mixture was stirred at r.t. for 24 h. The final crude product was filtered off and crystallized from methanol. Yield 2.25 g (78%). m.p. 207–210 °C. IR (nujol): ν 3421, 3238, 1594 cm−1. 1H-NMR (DMSO-d6) δ (ppm): 1.94–2.04 (m, 4H, 2 –CH2–CH2–CH2–), 3.08 (s, 9H, (CH3)3)N+–, 3.26 (br t, 2H –CH2–N+![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ), 3.36 (s, 6H, –(CH3)2N+–), 3.37–3.43 (m, 6H –CH2–N+
), 3.36 (s, 6H, –(CH3)2N+–), 3.37–3.43 (m, 6H –CH2–N+![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ), 6.70–8.02 (2d, 2H + 2H, ArH, J = 7.5 Hz), 7.37 (br s, 1H, –NH–). High-resolution ESI-MS (m/z): 162.1259 [C17H32N4O2]2+ (calcd 162.1257).
), 6.70–8.02 (2d, 2H + 2H, ArH, J = 7.5 Hz), 7.37 (br s, 1H, –NH–). High-resolution ESI-MS (m/z): 162.1259 [C17H32N4O2]2+ (calcd 162.1257).
Characterization of materials ACN1–ACN4
Preparation of composites 2·ACN3 and 10·ACN3
The material ACN3 (60 mg) was placed in a little beaker, wetted with 1 mL of methanol and suspended with 20 mL of an aqueous acetate buffer solution at pH 4.4. At the same time, the chosen guest (12 mg) was dissolved with 1 mL of a DMSO/methanol mixture 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, and the solution was transferred into a microsyringe. Then, the guest solution was slowly added (at the rate of 5 μL min−1) under vigorous stirring into the suspension of the polymer. After the addition was completed, the suspension was further kept under stirring overnight. The solid was then collected by centrifugation, filtered off under vacuum, washed with 3 portions (1 mL each) of cold methanol, and finally dried under vacuum at 50 °C overnight. In order to determine the amount of guest present in the composite, an accurately weighted amount (ca. 2 mg) of composite was placed in a vial, suspended with 1 mL of methanol, and sonicated for 10 min. The vial was then subjected to centrifugation, and the supernatant liquor was carefully pipetted. The procedure was repeated 4 further times, and the collected methanolic extracts were diluted with acetate aqueous buffer (pH 4.4) up to 20 mL. The concentration of the resulting solution was easily determined spectrophotometrically; thus, the amount of guest present in the composite was calculated by few trivial passages, resulting as large as 11% w/w for 2 ACN3 and 5% for 10·ACN3.
1 v/v, and the solution was transferred into a microsyringe. Then, the guest solution was slowly added (at the rate of 5 μL min−1) under vigorous stirring into the suspension of the polymer. After the addition was completed, the suspension was further kept under stirring overnight. The solid was then collected by centrifugation, filtered off under vacuum, washed with 3 portions (1 mL each) of cold methanol, and finally dried under vacuum at 50 °C overnight. In order to determine the amount of guest present in the composite, an accurately weighted amount (ca. 2 mg) of composite was placed in a vial, suspended with 1 mL of methanol, and sonicated for 10 min. The vial was then subjected to centrifugation, and the supernatant liquor was carefully pipetted. The procedure was repeated 4 further times, and the collected methanolic extracts were diluted with acetate aqueous buffer (pH 4.4) up to 20 mL. The concentration of the resulting solution was easily determined spectrophotometrically; thus, the amount of guest present in the composite was calculated by few trivial passages, resulting as large as 11% w/w for 2 ACN3 and 5% for 10·ACN3.
Adsorption/sequestration tests
Stock solutions 50 μM of the different guests were prepared in aqueous buffers at the desired pH values. Samples were prepared by mixing 2 mL of guest solution with a carefully weighted amount (4.00 ± 0.05 mg) of material. The samples were mechanically shaken at room temperature for 60 min, and then centrifuged for 15 min. The supernatant liquor was carefully pipetted and analysed spectrophotometrically to determine the percent amount of guest left in solution at equilibrium.Conclusions
The synthesis and characterization of new polyamino-cyclodextrin nanosponges is described. The materials can be easily obtained by a simple nucleophilic displacement reaction approach. They show a quite compact structure and a fair thermal stability, as well as a good hydrophilic character and the ability to be reversibly protonated by reaction with acidic or alkaline species, or by equilibration with a suitable buffer system. Sequestration tests positively assess their pH-dependent adsorption abilities towards variously structured organic guests. Moreover, composites can be easily prepared with good loadings. Solid state FSLG-2D NMR investigations provided unambiguous evidence on how the guest molecules actually interact with the cyclodextrin monomer subunits.Two final points are worth stressing in our opinion. First, because of the exploitation of an electrophilic cyclodextrin synthon, the synthetic approach we used to obtain our materials constitutes a sort of “change of paradigm” in the synthesis of cyclodextrin-based nanosponges with respect to most up-to-date literature, this, of course, opens new perspectives in the field. Finally, our nanosponges undoubtedly appear as promising “pH-smart” materials, for possible applications as drug carrier/delivery systems or in environment remediation devices.
Acknowledgements
Solid-state NMR spectra were acquired at the Centro Grandi Apparecchiature – UniNetLab – Università di Palermo funded by P.O.R. Sicilia 2000–2006, Misura 3.15 Quota Regionale; Prof. E. Caponetti and Dr A. Spinella are gratefully acknowledged for useful discussion and collaboration. The DSC instrumentation was kindly made available within the Project “PlASS” (Platform for Agrofood Science and Safety) funded by MIUR (project code: PONa3_00053); Prof. A. Pace is gratefully acknowledged.Notes and references
- (a) R. Cavalli, F. Trotta and W. Tumiatti, J. Inclusion Phenom. Macrocyclic Chem., 2006, 56, 209 CrossRef CAS; (b) F. Trotta and R. Cavalli, Compos. Interfaces, 2009, 16, 39 CrossRef CAS; (c) S. Subramanian, A. Singireddy, K. Krishnamoorthy and M. Rajappan, J. Pharm. Pharm. Sci., 2012, 15, 103 CAS.
- (a) F. Trotta, M. Zanetti and R. Cavalli, Beilstein J. Org. Chem., 2012, 8, 2091 CrossRef CAS PubMed; (b) A. Vyas, S. Saraf and S. Saraf, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 23 CrossRef CAS; (c) S. Swaminathan, P. R. Vavia, F. Trotta, R. Cavalli, S. Tumbiolo, L. Bertinetti and S. Coluccia, J. Inclusion Phenom. Macrocyclic Chem., 2013, 76, 201 CrossRef CAS.
- (a) L. Seglie, D. Spadaro, F. Trotta, M. Devecchi, M. L. Gullino and V. Scariot, Postharvest Biol. Technol., 2012, 64, 55 CrossRef CAS; (b) L. Seglie, K. Martina, M. Devecchi, C. Roggero, F. Trotta and V. Scariot, Postharvest Biol. Technol., 2011, 59, 200 CrossRef CAS; (c) L. Seglie, K. Martina, M. Devecchi, C. Roggero, F. Trotta and V. Scariot, Plant Growth Regul., 2011, 65, 505 CrossRef CAS.
- (a) B. B. Mamba, R. W. Krause, T. J. Malefetse, G. Gericke and S. P. Sithole, J. Water Supply: Res. Technol.--AQUA, 2009, 58, 299 CrossRef CAS; (b) D. P. Hashim, N. T. Narayanan, J. M. Romo-Herrera, D. A. Cullen, M. G. Hahm, P. Lezzi, J. R. Suttle, D. Kelkhoff, E. Muñoz-Sandoval, S. Ganguli, A. K. Roy, D. J. Smith, R. Vajtai, B. G. Sumpter, V. Meunier, H. Terrones, M. Terrones and P. M. Ajayan, Sci. Rep., 2012, 2(363), 1 Search PubMed; (c) B. B. Mumba, R. W. Krause, T. J. Malefetse and E. N. Nxumalo, Environ. Chem. Lett., 2007, 5, 79 CrossRef; (d) S. H. Mhlongo, B. B. Mamba and R. W. Krause, Phys. Chem. Earth, 2009, 34, 819 CrossRef.
- A. M. Layre, N. M. Gosselet, E. Renard, B. Sebille and C. Amiel, J. Inclusion Phenom. Macrocyclic Chem., 2002, 43, 311 CrossRef CAS.
- (a) J. Alongi, M. Pošković, A. Frache and F. Trotta, Polym. Degrad. Stab., 2010, 95, 2093 CrossRef CAS; (b) M. H. Mohamed, L. D. Wilson, D. Y. Pratt, R. Guo, C. Wu and J. D. Headley, Carbohydr. Polym., 2012, 87, 1241 CrossRef CAS; (c) F. Trotta, P. Ferruti, E. Ranucci, M. Veglia, C. Baggiani and C. Giovannoli, J. Inclusion Phenom. Macrocyclic Chem., 2007, 57, 657 CrossRef; (d) S. Berto, M. C. Bruzzaniti, R. Cavalli, D. Perrachon, E. Prenesti, F. Trotta, C. Sarzanini and W. Tumiatti, J. Inclusion Phenom. Macrocyclic Chem., 2007, 57, 631 CrossRef CAS; (e) F. Castiglione, V. Crupi, D. Majolino, A. Mele, W. Panzeri, B. Rossi, F. Trotta and V. Venuti, J. Inclusion Phenom. Macrocyclic Chem., 2013, 75, 247 CrossRef CAS.
- M. Ma and D. Q. Li, Chem. Mater., 1999, 11, 872 CrossRef CAS.
- P. Lo Meo, G. Lazzara, L. Liotta, S. Riela and R. Noto, Polym. Chem., 2014, 5, 4499 RSC.
- (a) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef; (b) V. V. Rostoftzef, L. G. Green, V. V. Forkin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef.
- (a) G. Pasparakis and M. Vamvakaki, Polym. Chem., 2011, 2, 1234 RSC; (b) A. K. Bajpai, J. Bajpai, R. Saini and R. Gupta, Polym. Rev., 2011, 51, 53 CrossRef CAS; (c) N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821 RSC; (d) S. K. Ahn, R. M. Kasi, S. C. Kim, N. Sharma and Y. X. Zhou, Soft Matter, 2008, 4, 1151 RSC; (e) H. J. Yoon and W. D. Yang, J. Mater. Chem., 2010, 20, 211 RSC; (f) D. Roy, J. N. Cambre and B. S. Sumerlin, Prog. Polym. Sci., 2010, 35, 278 CrossRef CAS.
- (a) G. H. Gao, M. J. Park, Y. Li, G. H. Im, J.-H. Kim, H. N. Kim, J. W. Lee, P. Jeon, O. Y. Bang, J. H. Lee and D. S. Lee, Biomaterials, 2012, 33, 9157 CrossRef CAS PubMed; (b) D. L. Gin and W. Gu, Adv. Mater., 2001, 13, 1407 CrossRef CAS; (c) H. C. Kang and Y. H. Bae, Adv. Funct. Mater., 2007, 17, 1263 CrossRef CAS; (d) M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398 CrossRef CAS PubMed; (e) S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081 CrossRef CAS PubMed.
- Z. Zeng, Y. Ai and S. Qian, Phys. Chem. Chem. Phys., 2014, 16, 2465 RSC.
- F. Fornasiero, J. B. In, S. Kim, H. G. Park, Y. Wang, C. P. Grigoropoulos, A. Noy and O. Bakajin, Langmuir, 2010, 26, 14848 CrossRef CAS PubMed.
- (a) J. Wu, K. C.-F. Leung, D. Benítez, J.-Y. Han, S. J. Cantrill, L. Fang and J. F. Stoddart, Angew. Chem., Int. Ed., 2008, 47, 7470 CrossRef CAS PubMed; (b) F. Cutrot, C. Romuald and E. Busseron, Org. Lett., 2008, 10, 3741 CrossRef PubMed.
- H. Liu, C. Wang, S. Zou, Z. Wei and Z. Tong, Langmuir, 2012, 28, 11017 CrossRef CAS PubMed.
- (a) Z. Li, D. L. Clemens, B.-Y. Lee, B. J. Dillon, M. A. Horwitz and J. I. Zink, ACS Nano, 2015, 9, 10778 CrossRef CAS PubMed; (b) K. Yang, H. Luo, M. Zeng, Y. Jiang, J. Li and X. Fu, ACS Appl. Mater. Interfaces, 2015, 7, 17399 CrossRef CAS PubMed; (c) H. P. Rim, K. H. Min, H. J. Lee, S. Y. Jeong and S. C. Lee, Angew. Chem., Int. Ed., 2011, 50, 8853 CrossRef CAS PubMed; (d) Y.-L. Zhao, Z. LI, S. Kabehie, Y. Y. Botros, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2010, 132, 13016 CrossRef CAS PubMed; (e) L. Dai, Q. Zhang, J. Li, X. Shen, C. Mu and K. Cai, ACS Appl. Mater. Interfaces, 2015, 7, 7357 CrossRef CAS PubMed.
- (a) P. Lo Meo, F. D'Anna, S. Riela, M. Gruttadauria and R. Noto, Org. Biomol. Chem., 2003, 1, 1584 RSC; (b) P. Lo Meo, F. D'Anna, S. Riela, M. Gruttadauria and R. Noto, Tetrahedron, 2004, 60, 9099 CrossRef CAS; (c) P. Lo Meo, F. D'Anna, S. Riela, M. Gruttadauria and R. Noto, Tetrahedron Lett., 2006, 60, 9099 CrossRef; (d) P. Lo Meo, F. D'Anna, S. Riela, M. Gruttadauria and R. Noto, Tetrahedron, 2007, 63, 9163 CrossRef CAS; (e) P. Lo Meo, F. D'Anna, S. Riela, M. Gruttadauria and R. Noto, Tetrahedron, 2009, 65, 2037 CrossRef CAS.
- (a) F. D'Anna, P. Lo Meo, S. Riela, M. Gruttadauria and R. Noto, Tetrahedron, 2001, 57, 6823–6827 CrossRef; (b) P. Lo Meo, F. D'Anna, S. Riela, M. Gruttadauria and R. Noto, Tetrahedron, 2002, 58, 6039 CrossRef CAS; (c) P. Lo Meo, F. D'Anna, S. Riela, M. Gruttadauria and R. Noto, Tetrahedron, 2009, 65, 10413 CrossRef CAS.
- (a) M. J. Laughton, B. Halliwell, P. J. Evans and J. R. Hoult, Biochem. Pharmacol., 1989, 38, 2859 CrossRef CAS PubMed; (b) L. M. Larocca, M. Piantelli, G. Leone, S. Sica, L. Teofili, P. B. Panici, G. Scambia, S. Manusco, A. Capelli and F. O. Ranelletti, Br. J. Haematol., 1990, 75, 489 CrossRef CAS PubMed; (c) A. W. Boots, H. Li, R. P. F. Schins, R. Duffin, J. W. M. Heemskerk, A. Bast and G. R. M. M. Haenen, Toxicol. Appl. Pharmacol., 2007, 222, 89 CrossRef CAS PubMed.
- (a) A. Valenzuela, C. Lagos, K. Schmidt and L. A. Videla, Biochem. Pharmacol., 1985, 34, 2209 CrossRef CAS PubMed; (b) K. Wellington and J. Blair, BioDrugs, 2001, 15, 465 CrossRef CAS PubMed; (c) C. Naveen, B. Subhendu, S. Rajveer and K. Jasbir, Int. J. Nutr., Pharmacol., Neurol. Dis., 2013, 3, 206 CrossRef.
- B. I. Gorin, R. J. Riopelle and G. R. J. Thatcher, Tetrahedron Lett., 1996, 37, 4647 CrossRef CAS.
- P. Lo Meo, F. D'Anna, M. Gruttadauria, S. Riela and R. Noto, Carbohydr. Res., 2012, 347, 32 CrossRef CAS PubMed.
- M. Russo, F. Armetta, S. Riela, D. Chillura Martino, P. Lo Meo and R. Noto, J. Mol. Catal. A: Chem., 2015, 408, 250 CrossRef.
- (a) D. D. Laws, H.-M. L. Bitter and A. Jerschov, Angew. Chem., Int. Ed., 2002, 41, 3096 CrossRef CAS; (b) M. J. Gidley and S. M. Bociek, J. Am. Chem. Soc., 1988, 110, 3820 CrossRef CAS; (c) W. G. Blann, C. A. Fyfe, J. R. Leyrla and C. S. Yannoni, J. Am. Chem. Soc., 1981, 103, 4030 CrossRef CAS.
- P. Conte, A. Piccolo, B. van Lagen, P. Buurman and M. A. Hemminga, Solid State Nucl. Magn. Reson., 2002, 21, 158 CrossRef CAS PubMed.
- W. Kolodziejski and J. Klinowski, Chem. Rev., 2002, 102, 613 CrossRef CAS PubMed.
- A. Naito, S. Ganapathy and C. A. McDowell, J. Magn. Reson., 1982, 48, 367 CAS.
- I. Borukhov, D. Andelman, R. Borrega, M. Cloitre, L. Leibler and H. Orland, J. Phys. Chem. B, 2000, 104, 11027 CrossRef CAS.
- (a) M. Furukawa, R. S. Farinato and E. Kokufuta, Colloid Polym. Sci., 2008, 286, 1425 CrossRef CAS; (b) H. Suzuki, B. Wang, R. Yoshida and E. Kokufuta, Langmuir, 1999, 15, 4283 CrossRef CAS.
- Y. Muroga and M. Nagasawa, Polym. J., 1986, 18, 15 CrossRef CAS.
- S. P. Sipin, M. K. Grachev, L. K. Vasyanina and E. E. Nifant’ev, Russ. J. Gen. Chem., 2006, 76, 1958 CrossRef CAS.
- M. D. Fernandez, M. J. Fernandez and M. Cobos, J. Mater. Sci., 2016, 51, 3628 CrossRef CAS.
- J. Pagacz, K. N. Raptopoulos, A. Leszczynka and K. Pielichowski, J. Therm. Anal. Calorim., 2016, 123, 1225 CrossRef CAS.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- M. Kruk, V. Antchshuk, M. Jaroniec and A. Sayari, J. Phys. Chem. B, 1999, 103, 10670 CrossRef CAS.
- M. V. Rekharsky, H. Yamamura, M. Kawai and I. Inoue, J. Org. Chem., 2003, 68, 5228 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |