Development of a novel biomimetic micro/nano-hierarchical interface for enhancement of osseointegration†
Abstract
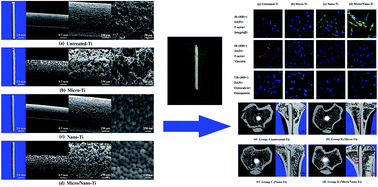
Surface modification of biomedical materials plays a significant role in enhancing in vivo osseointegration. In the present study, a biomimetic hierarchical titanium implant with micro- and nano-pores (Micro/Nano-Ti) was developed utilizing spark plasma sintering combined with electrochemical anodization. The surface morphology, phase composition, and wettability were characterized through scanning electron microscopy (SEM) and atomic force microscopy (AFM), X-ray diffraction (XRD), and contact angle (CA) measurement, respectively. Bone marrow cells were cultured, and the in vitro cell–material interactions were evaluated according to cell adhesion (SEM), cell viability (MTT), cell differentiation (ALP activity), mineralization (calcium deposition), focal protein adhesion (CLSM), and osteogenic factor expression (qRT-PCR). Additionally, the in vivo osseointegration properties were assessed by employing a tibia implantation rat model with subsequent histological analysis, micro-computed tomography, and biomechanical tests. The results showed that, in comparison with the untreated, Micro-Ti, and Nano-Ti implants, the Micro/Nano-Ti implant featured a hydrophilic surface (CA = 21 ± 2.1°) due to the presence of micro-pores (diameter: 30–100 μm) and nano-pores (diameter: 50–120 nm). Furthermore, the Micro/Nano-Ti implant greatly enhanced the in vitro cell–material interactions in terms of cell adhesion, viability, differentiation, mineralization, focal protein adhesion, and osteogenic factor expression, and accelerated osseointegration in vivo. The biomimetic hierarchical micro/nano-structure may represent an effective surface modification for potential application in the field of endosseous implants.

 Please wait while we load your content...
Please wait while we load your content...