Facile synthesis of gold–platinum dendritic nanostructures with enhanced electrocatalytic performance for the methanol oxidation reaction†
Abstract
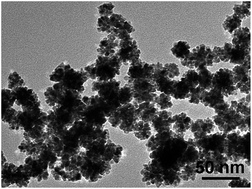
In this work, bimetallic gold–platinum (Au–Pt) dendritic nanoparticles with different Au/Pt ratios are synthesized via a surfactant-free wet-chemical route. The as-prepared products can be applied as a catalyst for the methanol oxidation reaction, and the results indicate that the Au–Pt dendritic nanoparticles with an Au/Pt ratio of 1 : 2.86 demonstrate the best catalytic performance. The surfactant-free method avoids the use of surfactants which can provide products with clean surfaces. Control experiments show that the catalytic performance of the Au–Pt samples is much higher than that of a poly(vinylpyrrolidone) (PVP) capped sample. Furthermore, the catalytic performance of the AuPt-2 sample with an Au/Pt ratio of 1 : 2.86 is much better than that of the commercial Pt/C catalyst and pure Pt nanoparticles. Thus, this work indicates that the Au–Pt dendritic nanoparticles may be used as efficient catalysts for direct methanol fuel cells.

 Please wait while we load your content...
Please wait while we load your content...