Polymer nanopillar array with Au nanoparticle inlays as a flexible and transparent SERS substrate
Abstract
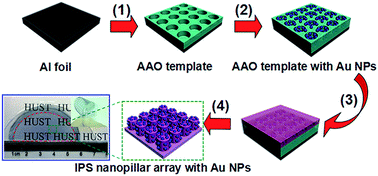
We report a facile and efficient way to fabricate a highly flexible, transparent and efficient surface-enhanced Raman scattering (SERS) substrate, in which Au nanoparticles (NPs) were embedded into the polymeric nanopillars via a nanoimprint lithography (NIL) method and using an anodic aluminum oxide (AAO) template. The obtained substrate exhibits prominent reproducibility and high sensitivity to Rhodamine 6G (R6G). Moreover, it possesses excellent transparency and flexibility. The SERS intensity acquired from these substrates almost remains constant after 200 bending cycles. Compared with traditional SERS substrates, this substrate represents a novel format with unique advantages, such as being highly flexible, transparent, lightweight, portable and easy to handle. More importantly, it can be scaled up for high-throughput production with low cost.

 Please wait while we load your content...
Please wait while we load your content...