Enhanced performance of a Pt-based three-way catalyst using a double-solvent method†
Jianjun Chena,
Fujin Huangb,
Wei Hub,
Guangxia Lib,
Lin Zhong*b and
Yaoqiang Chen*a
aCollege of Chemistry, Sichuan University, Chengdu, 610064, P. R. of China. E-mail: nic7501@scu.edu.cn; Fax: +86-28-85418451; Tel: +86-28-85418451
bCollege of Chemical Engineering, Sichuan University, Chengdu, 610064, P. R. of China. E-mail: zhonglin@scu.edu.cn
First published on 18th April 2016
Abstract
A new Pt three-way catalyst supported on CeO2–ZrO2–La2O3–Al2O3 via a double-solvent method was developed. For the conversion of a simulated compressed natural gas (CNG) vehicle exhaust, the catalyst showed a significant enhancement of the activity, which decreased the 100% conversion temperature (T100) for CH4 by about 46 °C compared with the catalyst prepared by conventional impregnation method.
In recent years, compressed natural gas (CNG) vehicles have attracted great attention due to their high fuel-efficiency and low exhaust emission. However, conversion of nitrogen oxide and unburned CH4 in CNG exhaust emission is still very challenging.1–4 To meet increasingly more stringent legislations (EURO 6, LEVII, and J-SULEV), there is a strong desire for development of catalysts with very high activity and durability for after-treatment of CNG vehicle exhaust emission.5,6 Recently, a number of catalysts supported on ordered porous materials such as SBA-15, nanotube and metal–organic framework (MOF) were prepared by a so-called double-solvent technique, and the catalysts showed enhanced activity in cyclohexanol oxidation and Fischer–Tropsch synthesis (FTS).7–11 However, the supports may suffer from either significant pore blockage or poor hydrothermal stability,12 which would lead to reduced catalytic performance. Al2O3-based composite supports may be candidates for addressing the problems as they normally have a 3D channel structure and superior hydrothermal stability.13–16 Nevertheless, few papers using the double-solvent method to support catalyst on these materials have been reported so far.
Herein, we report the use of the double-solvent method to support Pt on a CeO2–ZrO2–La2O3–Al2O3 (CZLA) composite, and a new Pt-based TWC was developed. The catalyst exhibited a superior catalytic performance in the elimination of a simulated CNG vehicle exhaust, compared with the catalyst prepared by conventional incipient wet impregnation method. The double-solvent technique can significantly decrease the 100% conversion temperature (T100) for CH4 by about 46 °C and the 100% N2-selectivity temperature from 427 °C to 380 °C.
The synthesis of the CeO2–ZrO2–La2O3–Al2O3 material with a C/Z/A molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and CZA/L mass ratio of 95
2 and CZA/L mass ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was carried out according to our previous work.17 The resulting CZLA powder was calcined at 900 °C for 3 h. The catalysts were synthesized using two methods. The first one was a typical double-solvent technique.7,8,18,19 Briefly, 10.000 g of CZLA powder was firstly suspended in 500 ml of dry n-hexane and the mixture was sonicated for 30 min until it became homogeneous. Then, a desired amount of Pt(NO3)2 precursor was dissolved in distilled water, whose quantity is corresponded to the pore volume of support determined by N2 adsorption. Finally, the hydrophilic Pt(NO3)2 solution was added dropwise to the hydrophobic n-hexane over a period of 20 min with constantly vigorous stirring. The resulting solution was continuously stirred for 3 h (300 rpm). The powder was recovered by careful filtration and dried in air at room temperature. The synthesized catalysts were further dried at 120 °C for 12 h, and then calcined at 550 °C for 3 h. The resulted catalyst was referred to as Pt-ds. For comparison, the second catalyst was prepared by the conventional incipient wet impregnation method and was denoted as Pt-im. The catalyst powders were ball milled to get homogeneous slurry. Then, the resulting slurry was coated onto cylindrical cordierite monoliths (Corning, USA, 400 cells per in.2, 2.5 cm3), then dried at 120 °C overnight and calcined at 550 °C in air for 3 h to obtain the monolithic catalysts.20 Both catalysts had the same Pt loading amount, with a content of 1.8 wt% relative to the amount of CZLA powder. The loading of washcoat was kept at about 160 g L−1 with a relative error of ±2%.
5 was carried out according to our previous work.17 The resulting CZLA powder was calcined at 900 °C for 3 h. The catalysts were synthesized using two methods. The first one was a typical double-solvent technique.7,8,18,19 Briefly, 10.000 g of CZLA powder was firstly suspended in 500 ml of dry n-hexane and the mixture was sonicated for 30 min until it became homogeneous. Then, a desired amount of Pt(NO3)2 precursor was dissolved in distilled water, whose quantity is corresponded to the pore volume of support determined by N2 adsorption. Finally, the hydrophilic Pt(NO3)2 solution was added dropwise to the hydrophobic n-hexane over a period of 20 min with constantly vigorous stirring. The resulting solution was continuously stirred for 3 h (300 rpm). The powder was recovered by careful filtration and dried in air at room temperature. The synthesized catalysts were further dried at 120 °C for 12 h, and then calcined at 550 °C for 3 h. The resulted catalyst was referred to as Pt-ds. For comparison, the second catalyst was prepared by the conventional incipient wet impregnation method and was denoted as Pt-im. The catalyst powders were ball milled to get homogeneous slurry. Then, the resulting slurry was coated onto cylindrical cordierite monoliths (Corning, USA, 400 cells per in.2, 2.5 cm3), then dried at 120 °C overnight and calcined at 550 °C in air for 3 h to obtain the monolithic catalysts.20 Both catalysts had the same Pt loading amount, with a content of 1.8 wt% relative to the amount of CZLA powder. The loading of washcoat was kept at about 160 g L−1 with a relative error of ±2%.
Fig. 1a shows the N2 adsorption–desorption isotherms of the samples. All samples exhibit type IV isotherms with H2 hysteresis loop according to the IUPAC classification.21 This type isotherm is characteristic properties of an irregular mesoporous material.15 The BJH method was employed to calculate the pore size distributions (PSD) of the support and catalysts (inset in Fig. 1a). The support and catalysts have almost the same pore size distributions within the range of 2–20 nm. The specific surface areas of Pt-ds and Pt-im decreased by 18.1% and 22.7%, respectively, compared with that of the support (Table 1). Fig. 1b shows the powder X-ray diffraction (XRD) patterns of the Pt containing catalysts, which are very similar to those of the supports. The main diffraction peaks of all samples are consistent with the characteristic peaks of tetragonal Ce0.5Zr0.5O2 (PDF-ICDD38-1436).22 There are no separated Al2O3 or La2O3 peak, which implies that Al3+ or La3+ may enter into the Ce0.5Zr0.5O2 solid solution. Moreover, no diffraction peaks corresponding to Pt species are detected, which may be attributed to low Pt content or high dispersion of Pt species. It is concluded that the main textural property and the crystal phase structure of the support are retained upon Pt-loading via both methods.
 | ||
| Fig. 1 (a) The nitrogen adsorption–desorption isotherms and pore-size distribution (inset figure) of the samples; (b) the XRD patterns of the support and catalysts. | ||
| Sample | SBET (m2 g−1) | V (ml g−1) | R (nm) |
|---|---|---|---|
| Support | 110.9 | 0.36 | 6.5 |
| Pt-im | 85.1 | 0.29 | 6.9 |
| Pt-ds | 90.0 | 0.29 | 6.5 |
Table 2 shows the Pt dispersion, active metal surface area23 (MSA) and average Pt particle size on the catalyst surface determined by CO chemisorption. Compared to the conventional incipient wet impregnation method, the double-solvent technique can significantly increase Pt dispersion from 21.2% to 35.5% and active MSA by about 60%. Moreover, the calculated size of Pt-ds is much smaller than that of Pt-im. Thus, enhanced activity for catalytic removal of pollutants in vehicle exhaust emissions may be obtained due to large quantities of highly dispersed Pt, the size effect and structure sensitivity of Pt nanoparticles.24,25
The morphology and microstructure of the as synthesized catalysts were analysed by Transmission electron microscopy (TEM) experiments (Fig. 2). The presence of Pt nanoparticles is confirmed by d-spacing measurements, and Pt nanoparticles are crystalline with the spacing of 0.27 nm and 0.24 nm corresponding to Pt(1 1 0) and Pt(1 1 1), respectively.7,27 It demonstrates that the smaller Pt nanoparticles (about 2–3 nm) of the Pt-ds catalyst are more homogeneously dispersed than those bigger ones (about 5–6 nm) of the Pt-im catalyst, which is consistent with the CO-chemisorption results (Table 2).
In order to evaluate the reducibility of the samples, H2-temperature programmed reduction (TPR) experiments were carried out (Fig. 3a). For the support, the reduction peak (about 508 °C) can be ascribed to the simultaneous reduction of the surface and bulk oxygen owing to the high oxygen mobility in CeO2–ZrO2 solid solution. The TPR profiles of Pt-im and Pt-ds catalysts are significantly different from that of the support. For Pt-im catalyst, the reduction peak with a high intensity appears at 147 °C and a shoulder appears at 256 °C, which can be associated with the reduction of PtOx species and the interaction between PtOx species and the support, respectively.28 Additionally, the reduction peak maxima at ca. 404 °C can also be attributed to the reduction of surface CeO2.29 For Pt-ds catalyst, the main peak located at 133 °C can be ascribed to the reduction of PtOx species and the shoulder peak at 223 °C can be assigned to the reduction of surface CeO2 having interaction with Pt. Note that the reduction peaks of Pt-ds shift remarkably to lower temperatures, which may be due to the fact that Pt-ds has smaller Pt particles, better dispersion and stronger interaction between Pt and the support. Thus, Pt-ds can provide better reducibility than Pt-im.
 | ||
| Fig. 3 (a) H2-TPR profiles of the samples; (b) OSC values of the support and catalysts obtained by the O2 pulses at different temperatures. | ||
Fig. 3b displays the oxygen storage capacity (OSC) amount of the samples at 200, 300, 400, 500, and 600 °C, respectively. For all the samples, the OSC values increase with increment of temperature. However, compared with the support, the catalysts display better OSC performance because of the more oxygen vacancies generated by the Pt species.30 The generation may follow a route of the reduction of support by spilt H atoms from Pt to the support or a route of the electron transfer from the conduction band of support to the Pt phase.30–33 Interestingly, Pt-ds exhibits higher OSC value than that of Pt-im at the same temperature, which could be due to the fact that Pt-ds have the larger number of oxygen vacancies and the stronger interaction between Pt species and the support.26,30 Thus, Pt-ds may display higher activity in TWC application. However, it is important to note that the transient rate of labile oxygen supply of support to the Pt surface can also affect the TWC performance of the Pt catalysts.33–35
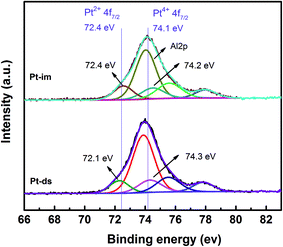
The chemical state of Pt and the surface composition of the catalysts were studied via the X-ray photoelectron spectroscopy (XPS) technique (Fig. 4). The method of curve fitting for the XPS Pt 4f spectra is according to the reported method.36 It is found that Platinum nanoparticles of both catalysts exist mainly in an oxide form after being calcined in air37 and the relative abundance (Table 3) of PtO2 (45.1%) on Pt-ds is higher than that on Pt-im (39.6%). The result indicates that the double-solvent method is favorable to oxidize Pt species, which could be due to the smaller platinum particles for Pt-ds that can be oxidized more easily in ambient air.38 Similarly, Pt-ds with smaller PtOx particles should also be reduced more easily under the flow of a reducing gas,37 which is confirmed by the results of H2-TPR. Furthermore, for Pt-ds, the shift of the XPS Pt2+ 4f7/2 spectrum (0.3 eV) relative to the reference line36 is higher than that of Pt-im (0.0 eV), which can be due to the smaller particles of Pt-ds with stronger metal–support interaction (SMSI).36 Therefore, the results show that the double-solvent method can enhance the reducibility of Pt and the interaction between Pt and the support which are beneficial for the improvement of the catalytic activity for Pt-ds.
| Catalyst | Pt species (%) | Pt2+ 4f7/2 (eV) | Pt4+ 4f7/2 (eV) | ||
|---|---|---|---|---|---|
| Pt0 | PtO | PtO2 | |||
| Pt-im | 0 | 60.4 | 39.6 | 72.4 | 74.2 |
| Pt-ds | 0 | 54.9 | 45.1 | 72.1 | 74.3 |
The conversions of CH4 (a), NO (b) and CO (c), and selectivity of N2 (d) over Pt-im and Pt-ds are presented in Fig. 5. The conversions of CH4 and CO for both catalysts increase with temperature. For NO, however, it mainly reacts with CO at low temperature and with CH4 at high temperature, and there is a balance between the two reactions.39 Pt-ds shows 100% CH4 conversion (T100) at 396 °C, while for Pt-im, the value increases to 442 °C. Moreover, the ΔT (ΔT = T100 − T50) value of CH4 for Pt-ds (28 °C) is smaller than that for Pt-im (40 °C), suggesting that CH4 over Pt-ds can more easily reach complete conversion after light-off. Interestingly, for the conversion activity of NO and CO, the performance of Pt-ds is also better than that of Pt-im. It is worth noting that a similar behavior is observed for the selectivity towards N2. Pt-ds has a superior N2-selectivity, which is up to 100% at 380 °C. However, for Pt-im, the other N-containing product species (NO2, N2O and NH3) can be formed (Fig. S1†) and a relatively poor N2-selectivity (60%) is obtained.
 | ||
| Fig. 5 Conversion curves of CH4 (a), NO (b) and CO (c), and selectivity of N2 (d) over Pt-im and Pt-ds. | ||
In summary, two catalysts, Pt-im and Pt-ds, were synthesized using the conventional incipient wet impregnation method and the double-solvent technique, respectively. The results of nitrogen adsorption–desorption, XRD, CO-chemisorption, TEM, H2-TPR, OSC and XPS indicate that Pt-ds has higher dispersion and better reducibility of Pt species and stronger interaction between these species and the support. Thus, Pt-ds exhibits a significant enhancement of the activity in the elimination of NO, CO and CH4 from a simulated CNG vehicle exhaust, compared with Pt-im. It is found that all three pollutants can be converted completely over Pt-ds and the N2-selectivity can be up to 100% at a low temperature (about 400 °C). Our work may provide a facile but efficient method to develop highly efficient catalysts for the conversion of the vehicle exhaust. We note that a future suitable TWC for CNG exhausts emissions would be Pd-based catalyst due to its significantly lower cost compared to Pt-based one. Further work on developing the Pd-based TWC catalyst via double-solvent method is underway in our lab.
Acknowledgements
This work is sponsored by the National Hi-tech Research and Development Program of China (863 Program, 2015AA034603 and 2013AA065304), and Sichuan Province science and technology support program (2014SZ0143 and 2015GZ0054).Notes and references
- P. Gélin and M. Primet, Appl. Catal., B, 2002, 39, 1–37 CrossRef.
- M. Hussain, F. A. Deorsola, N. Russo, D. Fino and R. Pirone, Fuel, 2015, 149, 2–7 CrossRef CAS.
- D. Fino, N. Russo, G. Saracco and V. Specchia, Prog. Solid State Chem., 2007, 35, 501–511 CrossRef CAS.
- N. Russo, P. Palmisano and D. Fino, Chem. Eng. J., 2009, 154, 137–141 CrossRef CAS.
- A. Winkler, P. Dimopoulos, R. Hauert, C. Bach and M. Aguirre, Appl. Catal., B, 2008, 84, 162–169 CrossRef CAS.
- F. Klingstedt, H. Karhu, A. K. Neyestanaki, L. E. Lindfors, T. Salmi and J. Väyrynen, J. Catal., 2002, 206, 248–262 CrossRef CAS.
- A. Aijaz, A. Karkamkar, Y. J. Choi, N. Tsumori, E. Ronnebro, T. Autrey, H. Shioyama and Q. Xu, J. Am. Chem. Soc., 2012, 134, 13926–13929 CrossRef CAS PubMed.
- M. Imperor-Clerc, D. Bazin, M.-D. Appay, P. Beaunier and A. Davidson, Chem. Mater., 2004, 16, 1813–1821 CrossRef CAS.
- J. Taghavimoghaddam, G. P. Knowles and A. L. Chaffee, J. Mol. Catal. A: Chem., 2012, 358, 79–88 CrossRef CAS.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- J. van der Meer, I. Bardez-Giboire, C. Mercier, B. Revel, A. Davidson and R. Denoyel, J. Phys. Chem. C, 2010, 114, 3507–3515 CAS.
- J. Taghavimoghaddam, G. P. Knowles and A. L. Chaffee, J. Mol. Catal. A: Chem., 2013, 377, 115–122 CrossRef CAS.
- X. Yao, C. Tang, F. Gao and L. Dong, Catal. Sci. Technol., 2014, 4, 2814 CAS.
- M. H. Yao, R. J. Baird, F. W. Kunz and T. E. Hoost, J. Catal., 1997, 166, 67–74 CrossRef CAS.
- J. Wang, J. Wen and M. Shen, J. Phys. Chem. C, 2008, 112, 5113–5122 CAS.
- N. S. Priya, C. Somayaji and S. Kanagaraj, J. Nanopart. Res., 2014, 16, 2214 CrossRef.
- X. Zhang, E. Long, Y. Li, J. Guo, L. Zhang, M. Gong, M. Wang and Y. Chen, J. Nat. Gas Chem., 2009, 18, 139–144 CrossRef CAS.
- I. Lopes, N. El Hassan, H. Guerba, G. Wallez and A. Davidson, Chem. Mater., 2006, 18, 5826–5828 CrossRef CAS.
- Q.-L. Zhu, J. Li and Q. Xu, J. Am. Chem. Soc., 2013, 135, 10210–10213 CrossRef CAS PubMed.
- L. Lan, S. Chen, Y. Cao, S. Wang, Q. Wu, Y. Zhou, M. Huang, M. Gong and Y. Chen, J. Mol. Catal. A: Chem., 2015, 410, 100–109 CrossRef CAS.
- K. S. W. SING, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- G. Vlaic, R. Di Monte, P. Fornasiero, E. Fonda, J. Kašpar and M. Graziani, J. Catal., 1999, 182, 378–389 CrossRef CAS.
- S. B. Kang, S. J. Han, S. B. Nam, I.-S. Nam, B. K. Cho, C. H. Kim and S. H. Oh, Top. Catal., 2013, 56, 298–305 CrossRef CAS.
- J. Garciacortes, J. Catal., 2003, 218, 111–122 CrossRef CAS.
- S. Vajda, M. J. Pellin, J. P. Greeley, C. L. Marshall, L. A. Curtiss, G. A. Ballentine, J. W. Elam, S. Catillon-Mucherie, P. C. Redfern, F. Mehmood and P. Zapol, Nat. Mater., 2009, 8, 213–216 CrossRef CAS PubMed.
- J. Fan, X. Wu, X. Wu, Q. Liang, R. Ran and D. Weng, Appl. Catal., B, 2008, 81, 38–48 CrossRef CAS.
- J. Fan, X. Wu, L. Yang and D. Weng, Catal. Today, 2007, 126, 303–312 CrossRef CAS.
- Y. Zheng, Y. Zheng, Y. Xiao, G. Cai and K. Wei, Catal. Commun., 2013, 39, 1–4 CrossRef CAS.
- L. Liu, Y. Cao, W. Sun, Z. Yao, B. Liu, F. Gao and L. Dong, Catal. Today, 2011, 175, 48–54 CrossRef CAS.
- C. Bozo, N. Guilhaume and J.-M. Herrmann, J. Catal., 2001, 203, 393–406 CrossRef CAS.
- M.-F. Luo and X.-M. Zheng, Appl. Catal., A, 1999, 189, 15–21 CrossRef CAS.
- N. W. Hurst, S. J. Gentry, A. Jones and B. D. McNicol, Catal. Rev., 2007, 24, 233–309 Search PubMed.
- C. N. Costa, S. Y. Christou, G. Georgiou and A. M. Efstathiou, J. Catal., 2003, 219, 259–272 CrossRef CAS.
- A. M. Efstathiou and S. Y. Christou, Catal. Sci. Series, in Catalysis by Ceria and Related Materials, ed. A. Trovarelli and P. Fornasiero, Imperial College Press, London, 2nd edn, 2013, vol. 12, ch. 3, pp. 139–222 Search PubMed.
- P. S. Lambrou, C. N. Costa, S. Y. Christou and A. M. Efstathiou, Appl. Catal., B, 2004, 54, 237–250 CrossRef CAS.
- L. Olsson, J. Catal., 2002, 210, 340–353 CrossRef CAS.
- Z. Yang, N. Zhang, Y. Cao, M. Gong, M. Zhao and Y. Chen, Catal. Sci. Technol., 2014, 4, 3032 CAS.
- E. Fridell, A. Amberntsson, L. Olsson, A. Grant and M. Skoglundh, Top. Catal., 2004, 30–31, 143–146 CrossRef.
- M. Salaün, A. Kouakou, S. Da Costa and P. Da Costa, Appl. Catal., B, 2009, 88, 386–397 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06285g |
| This journal is © The Royal Society of Chemistry 2016 |