Solvothermally synthesized nanoporous hypercrosslinked polyaniline: studies of the gas sorption and charge storage behavior†
Vivek Sharmaa,
Santimoy Khilarib,
Debabrata Pradhanb and
Paritosh Mohanty*a
aDepartment of Chemistry, Indian Institute of Technology Roorkee, Roorkee-247667, Uttarakhand, India. E-mail: paritosh75@gmail.com; Tel: +91-1332-284859
bMaterials Science Centre, Indian Institute of Technology Kharagpur, Kharagpur-721302, W. B., India
First published on 6th June 2016
Abstract
A facile solvothermal method has been developed for the synthesis of high surface area nanoporous hypercrosslinked polyaniline (HCPANISs). A series of samples have been prepared by varying the hypercrosslinker content and reaction temperature. The maximum specific surface area (SABET) of 711 m2 g−1 with the pore size distribution centered at 0.64 nm was measured for the sample synthesized with 1 mL diiodomethane (hypercrosslinker) at 160 °C (HCPANIS-1 mL-160 °C). This specimen further exhibited a CO2 and CH4 uptake of 14.7 and 1.8 wt%, respectively, at 0 °C and 1 bar. It also showed the H2 storage capacity of 1.18 wt% at −196 °C and 1 bar. HCPANIS-1 mL-160 °C was further investigated for electrochemical supercapacitor applications. The maximum specific capacitance calculated was 580 F g−1 at a scan rate of 3 mV s−1 with 68% retention capacity over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a current density of 10 A g−1.
000 cycles at a current density of 10 A g−1.
Introduction
Polyaniline (PANI) is one of the most extensively investigated organic polymers since its discovery in 1835 as “aniline black”.1 It's structure was correctly predicted only in 1862 by Letheby.2 The electrical conducting behavior of PANI was first reported by MacDiarmid in 1985.3 After this, extensive research has been carried out on studying various properties of PANI and its composites.4–18 The popularity of PANI in various research areas could easily be seen by the large number of research articles (around 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) published in the last decade.19 Amongst them, the applications related to the electrical properties such as electrochemical supercapacitors,4–8 anticorrosive coatings,9 light emitting diodes,10 gas sensors,11–13 humidity sensors,14 and actuators15 etc. have been investigated extensively. One of the interesting properties of PANI is the change in its behavior (and form) in presence of acid and base.13,14 The doping–dedoping and controlled redox behavior has made PANI an interesting candidate for sensors, actuators, and supercapacitor applications.4–14 The performance and efficiency of PANI for the above mentioned applications could be enhanced by synthesizing PANI with high specific surface area.5,16 In general, the specific surface area of PANI has been increased by crosslinking and hypercrosslinking the PANI chains.5,16–18 The crosslinking of PANI was carried out mostly by thermal treatment,18 however, microwave assisted methods, conventional methods have been used for hypercrosslinking through N-alkylation chemistry and Friedel–craft alkylation reaction.5,16,17 A few other methods that have been adopted to increase the surface area of PANI were emulsion process,20 bubble induced mechanism,21 and template assisted method.6
000) published in the last decade.19 Amongst them, the applications related to the electrical properties such as electrochemical supercapacitors,4–8 anticorrosive coatings,9 light emitting diodes,10 gas sensors,11–13 humidity sensors,14 and actuators15 etc. have been investigated extensively. One of the interesting properties of PANI is the change in its behavior (and form) in presence of acid and base.13,14 The doping–dedoping and controlled redox behavior has made PANI an interesting candidate for sensors, actuators, and supercapacitor applications.4–14 The performance and efficiency of PANI for the above mentioned applications could be enhanced by synthesizing PANI with high specific surface area.5,16 In general, the specific surface area of PANI has been increased by crosslinking and hypercrosslinking the PANI chains.5,16–18 The crosslinking of PANI was carried out mostly by thermal treatment,18 however, microwave assisted methods, conventional methods have been used for hypercrosslinking through N-alkylation chemistry and Friedel–craft alkylation reaction.5,16,17 A few other methods that have been adopted to increase the surface area of PANI were emulsion process,20 bubble induced mechanism,21 and template assisted method.6
Solvothermal is a widely used simple technique for the synthesis of various nanostructures of metals, metal oxides, metal–organic frameworks and polymers.22–25 Moreover, this method has been employed for the synthesis of nanoporous structures.22–25 There are a few reports on the crosslinking and hypercrosslinking of polymer chains using solvothermal method.25 Solvothermal is considered as an effective method because it has overcome the problems associated with high purity, high cost special equipment and low energy consumption.26 Further, this method provides a closed medium in which solvent remains in the liquid state at high temperature and also act as template, which leads to the formation of highly crosslinked network. The solvent in this method suppresses the segregation of highly crosslinked network effectively while increasing the local degree of polymerization. After completion of the reaction, very often monolith is obtained. The removal of the solvent from the monolith leaves permanent porosity in the crosslinked network.27
To the best of our knowledge, no report is available on the crosslinking and/or hypercrosslinking of PANI by the solvothermal process. In this report, for the first time, we demonstrate the solvothermal method as a potential and simple method to hypercrosslink PANI at a moderate temperature of 160 °C for 24 h using N,N-dimethylformamide (DMF) as solvent. The specific surface area of the emeraldine base (EBPANI) was increased from 44 m2 g−1 to 711 m2 g−1 (with pore diameter of 0.64 nm) upon hypercrosslinking in the present solvothermal method.
The specific surface area, presence of the ultramicropores and nitrogen with lone pair of electrons are known to be beneficial for the improved gas sorption properties of a material.28 For example, the nitrogen with lone pair of electrons acts as Lewis base and could interact with Lewis acidic gases such as CO2.28 As already documented in research articles and reports, the CO2 capture and separation from the flue gas stream could potentially solve the global warming effect.5,28–32 The presence of the ultramicroporosity could further be beneficial for the H2 storage application as H2 is considered as an ideal fuel for future generation due to its much higher energy density than gasoline and non polluting nature.5 Thus, finding suitable solid adsorbents both for CO2 capture and H2 storage remains a great challenge in the recent time and lead to extensive research.5,16 In order to store energy derived from the renewable sources such as solar, wind and tidal energy, it is highly desirable to find suitable storage system. In this context supercapacitor, a new type of energy storage device/system comes to the frontier of modern energy storage technology.4–8 Due to its high power density, long lifetime and short charging time as compared to lithium ion batteries, it becomes a promising energy storage system in the recent time.5,33,34 Various materials investigated for supercapacitor application are metal oxides, such as, RuO2, MnO2, NiO, Co3O4, V2O5 etc. and polymers, such as, PANI, polypyrrole (PPy), poly(3,4-ethylenedioxythiophen) (PEDOT).33,34 The advantages of PANI in supercapacitor application lies in its multiple redox states, rapid doping–dedoping during charge–discharge, reversible faradic reactions, low cost of raw materials, good environmental stability and most importantly high theoretical specific capacitance.4–8,33,34 In this context, the CO2 capture, H2 storage and supercapacitor applications of present hypercrosslinked nanoporous PANI with high surface area has immense scientific importance and has potential for the aforementioned applications as that demonstrated here.
Experimental
Materials
The reagents used in the synthesis of HCPANISs were aniline hydrochloride (Alfa Aesar, UK), ammonium persulfate (Himedia, India), ammonia solution (Merck, India), N,N-dimethylformamide (DMF) (Fisher Scientific, India), and diiodomethane (Himedia, India). These chemicals were used as received without any further purification.Synthesis of HCPANISs
The HCPANISs have been synthesized as shown in Scheme 1 by hypercrosslinking of EBPANI using diiodomethane as hypercrosslinker. The polymerization of aniline to form EBPANI was carried out by chemical oxidative polymerization using ammonium persulfate as free radical initiator as per our recent report.5 To optimize synthesis conditions in order to get best results, seven samples were prepared by varying the content of hypercrosslinker and reaction temperature. In a typical synthesis of HCPANISs, 0.11 g EBPANI was dissolved in 10 mL of DMF followed by ultrasonication for 1 h. To this solution, 1.1 g of anhydrous potassium carbonate was added and further sonicated for 30 min. To this reaction mixture, specified quantity (X mL) of diiodomethane was added. The resulting reaction mixture was transferred to a teflon lined autoclave and placed in hot air oven at Y °C for 24 h. The precipitate was collected by filtration and refluxed in DMF for 3 days. The final products were designated as HCPANIS-X mL-Y °C. | ||
| Scheme 1 Reaction scheme for the synthesis of HCPANISs (3D structures were made with the help of ACD/ChemSketch software). | ||
Measurements
The FTIR spectra were recorded using PerkinElmer FTIR Spectrometer Spectrum Two in the wavenumber range of 4000 to 450 cm−1. The 13C CPMAS-NMR spectrum was recorded using JEOL RESONANCE ECX-400. The TGA/DTG analysis was performed using EXSTAR TG/DTA 6300 instrument with a heating rate of 10 °C min−1 in air. The XRD patterns were obtained with Rigaku, Ultima-IV X-ray diffractometer in the 2θ range 5–70° using Cu Kα radiation (λ = 1.5405 Å). FESEM images were obtained using MIRA3 TESCAN at suitable operating voltage. Prior to the analysis, the samples were gold coated using standard sputtering technique. TEM images were taken on JEM-2100 HRTEM, JEOL with an operating voltage of 200 kV by dispersing the specimen in ethanol followed by placing a drop on the carbon coated copper grids. The textural properties and gas sorption studies were done using Autosorb-iQ2 (Quantachrome Instruments, USA). Before the analysis, the samples were degassed in vacuum for 6 h at 120 °C with a heating rate of 5 °C min−1.All the electrochemical measurements were carried out in a CH Instruments 660D electrochemical workstation. The electrode setup was consisting of active material slurry coated glassy carbon electrode, saturated calomel electrode (SCE) and Pt wire as working, reference, and counter electrodes, respectively. The active material slurry was prepared by dispersing the HCPANIS sample in ethanol followed by addition of polytetrafluoroethylene (PTFE) binder. The geometric surface area of glassy carbon electrode used in the present study was 0.07 cm2. The loading of active material on glassy carbon electrode was estimated to be 0.29 mg cm2. The cyclic voltammograms (CVs) and galvanostatic charge discharge (GCD) measurements were carried out in 1 M H2SO4. The CVs were taken in different scan rate in a potential window of 0.0 to 0.9 V with respect to SCE. The GCD was studied at 2, 5, 8 and 10 A g−1 current density within the potential range of 0.0 to 0.9 V vs. SCE. The cyclic stability was checked for up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 current density within the same potential window.
000 cycles at 10 A g−1 current density within the same potential window.
Results and discussion
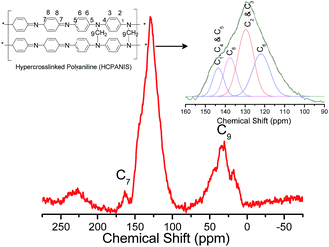
The formation of HCPANIS and hypercrosslinking of EBPANI as shown in Scheme 1 was investigated and confirmed by FTIR and 13C CPMAS NMR spectroscopy. The appearance of strong bands around 2900 and 1050 cm−1 in the FTIR spectrum of HCPANIS-1 mL-160 °C as shown in Fig. S1b† were attributed to the stretching and bending vibrations of CH2 groups that confirms the hypercrosslinking.5,35 These bands were absent in the spectrum of EBPANI (Fig. S1a†). The slight up-shifting of the 1500 and 1600 cm−1 bands of EBPANI appeared for HCPANIS-1 mL-160 °C, which is due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching deformation of benzenoid and quinoid rings, respectively, further indicating the hypercrosslinking.5,16,35 The observation of band around 1235 cm−1 is due to aliphatic C–N bond further confirming the hypercrosslinking of EBPANI.5,36 These observations in the FTIR spectrum of HCPANIS-1 mL-160 °C are in good agreement with the reported literature on N-alkylation of polyaniline.5,16,35 Similar observations were obtained for other specimens indicating successful hypercrosslinking of EBPANI chains (Fig. S2†). A broad signal centered at 30 ppm in the 13C CPMAS NMR spectrum in Fig. 1 attributed to the hypercrosslinked CH2 group confirming the formation of the HCPANIS.5,37 In addition to this, the signals for the different carbons of the benzenoid and quinoid rings (shown in the inset of Fig. 1) were observed around 121, 129, 137, 143 and 163 ppm, and were matched well with the reported values.5,38
C stretching deformation of benzenoid and quinoid rings, respectively, further indicating the hypercrosslinking.5,16,35 The observation of band around 1235 cm−1 is due to aliphatic C–N bond further confirming the hypercrosslinking of EBPANI.5,36 These observations in the FTIR spectrum of HCPANIS-1 mL-160 °C are in good agreement with the reported literature on N-alkylation of polyaniline.5,16,35 Similar observations were obtained for other specimens indicating successful hypercrosslinking of EBPANI chains (Fig. S2†). A broad signal centered at 30 ppm in the 13C CPMAS NMR spectrum in Fig. 1 attributed to the hypercrosslinked CH2 group confirming the formation of the HCPANIS.5,37 In addition to this, the signals for the different carbons of the benzenoid and quinoid rings (shown in the inset of Fig. 1) were observed around 121, 129, 137, 143 and 163 ppm, and were matched well with the reported values.5,38
The microstructure of the HCPANISs was investigated by both FESEM and TEM. Nearly spherical or elongated particles of size in the range of 50–200 nm in the agglomerated form can be seen in the FESEM images of the specimens in Fig. 2. There is no major change in surface morphology and size of particles by changing either reaction temperature or quantity of hypercrosslinker. Fig. 2h shows a representative TEM image of HCPANIS-1 mL-160 °C which corroborates the FESEM study. Interestingly, these particles are highly porous in nature as can be seen in the high magnification TEM image in Fig. 2i. As expected the specimens were amorphous in nature as confirmed from XRD (Fig. S3†) and selected area electron diffraction pattern (SAED) (inset of Fig. 2i). Furthermore, the thermal stability of HCPANIS-1 mL-160 °C in air was investigated by TGA/DTG analysis (Fig. S4†) and observed that the material was thermally stable up to a temperature of ≈400 °C.
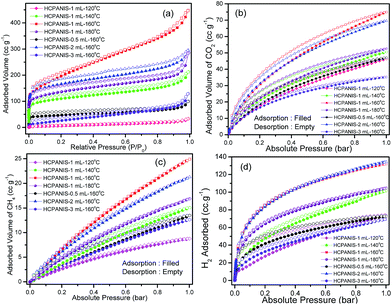
The textural properties of the HCPANISs were studied by N2 sorption analysis at −196 °C (Fig. 3a). The N2 sorption isotherms of the specimens are almost similar and exhibited type-I isotherm with a rapid uptake at low relative pressure range (P/P0 < 0.1) and small hysteresis extended towards relative lower pressure range indicating the presence of micropores as well as mesopores in the specimens. This type of hysteresis is observed for the typical microporous materials, which could be due to the complex microporous structure.5,39 The maximum achieved specific surface area calculated by BET (SABET) and Langmuir (SALang.) equations were found to be 711 and 931 m2 g−1, respectively, for HCPANIS-1 mL-160 °C (Fig. S5†). SABET of other specimens were in the range of 30 to 607 m2 g−1 (Figs. S6–S11†). The total pore volume of HCPANIS-1 mL-160 °C calculated at relative pressure (P/P0) = 0.99 was found to be 0.69 cm3 g−1. The pore size distribution was estimated by DFT method using kernel slit/cylindrical pores, QSDFT adsorption branch (fitting error = 0.252%) as shown in Fig. S12,† suggesting that the specimen has hierarchical pores with the majority of pores in the microporous range (<2 nm). Moreover, some pores were also in the mesoporous range up to 6 nm. Detailed textural properties of HCPANISs are given in Table 1.
| Sample ID | SABET (m2 g−1) | SALang. (m2 g−1) | VTotal (cm3 g−1) |
|---|---|---|---|
| HCPANIS-1 mL-120 °C | 30 | 53 | 0.05 |
| HCPANIS-1 mL-140 °C | 401 | 492 | 0.33 |
| HCPANIS-1 mL-160 °C | 711 | 931 | 0.69 |
| HCPANIS-1 mL-180 °C | 489 | 661 | 0.44 |
| HCPANIS-0.5 mL-160 °C | 172 | 189 | 0.15 |
| HCPANIS-2 mL-160 °C | 607 | 698 | 0.46 |
| HCPANIS-3 mL-160 °C | 116 | 205 | 0.20 |
The high specific surface area, high pore volume, presence of ultramicroporosity as well as the Lewis basic nature of the amine groups present in the framework prompted us to study the CO2, CH4 uptake and hydrogen storage properties of HCPANISs. The HCPANIS-1 mL-160 °C exhibited maximum CO2 uptake capacity of 14.7 wt% and 8.7 wt% at 0 °C and 25 °C, respectively (Fig. 3b and S13a†). This CO2 uptake of HCPANIS-1 mL-160 °C at 0 °C is comparable with the CO2 uptake of some of the recently reported nanoporous polymers such as PECONFs (8.2–15.4 wt%),28 azo-COPs (8.4–11.4 wt%),40 azo-POFs (8.4–13.1 wt%),41 P-1 and P-2 (8.9 and 14.5 wt%),42 box-COPs (9.4–13.9 wt%),43 NPOFs (10.6–12.8 wt%),44 Cu/BF4/BIPLP-1 (11.3 wt%),45 SMPs (13.7–20.4 wt%),46 PBI-Ad-1 (17.3 wt%),47 and BILP-1 (18.8 wt%).48 The present material has high significance because of much larger CO2 uptake per m2 g−1 surface area (Table S1†). Quantitatively, the CO2 uptake of HCPANIS-1 mL-160 °C at 0 °C is 0.021 wt% m−2, which is higher than the several recent reports (Table S1†). Moreover, keeping in mind the specific surface area of HCPANIS-1 mL-160 °C, the CO2 capture capacity can be compared with other nanoporous materials such as metal–organic frameworks and activated carbon which have much higher specific surface areas.49,50 Although, there are several reports on much larger surface area MOFs and activated carbon, it remains a challenge to synthesize crosslinked polymers with high surface area as demonstrated here. The ease of synthesis, high thermal stability and the most importantly from the economic point of view the low cost of monomer makes HCPANI a better adsorbent than other reported materials.51 Although the best gas sorption properties reported till now are of metal–organic frameworks (MOFs) but the low thermal and chemical stability towards air and moisture hinders its industrial use.52 The CO2 capture capacity of some of the best recent reports is compared with the HCPANIS-1 mL-160 °C in Table S1.† Several of these materials such as SMPs-7,46 PPF-1,51 and CPOP-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 and are synthesized from expensive precursors than that of EBPANI employed in the present work. HCPANIS-1 mL-120 °C, HCPANIS-1 mL-140 °C, HCPANIS-1 mL-180 °C, HCPANIS-0.5 mL-160 °C, HCPANIS-2 mL-160 °C and HCPANIS-3 mL-160 °C also showed noteworthy CO2 uptake of 9.1 wt%, 9.9 wt%, 10.3 wt%, 9.2 wt%, 13.6 wt% and 6.9 wt% at 0 °C, respectively (Fig. 3b). The CO2 sorption isotherms measured at both 0 °C and 25 °C are almost reversible with minor hysteresis which extends from the high pressure range to the low pressure range (Fig. 3b and S13a†). This indicates that the interaction was neither purely physisorption nor purely chemisorption in nature. This was further confirmed from the isosteric heat of adsorption (Qst) in the range of 27.62 kJ mol−1 to 35.17 kJ mol−1 calculated using Clausius–Clapeyron equation (Fig. S14†). The CH4 uptake of HCPANIS-1 mL-160 °C were observed to be 1.8 wt% and 0.86 wt% at 0 °C and 25 °C, respectively, as shown in Fig. 3c and S13b.† Both the isotherms were completely reversible and the calculated Qst at zero coverage was estimated to be 21.38 kJ mol−1 (Fig. S15†). The details of methane adsorption for other synthesized specimens are shown in Table 2. The CO2/N2, CH4/N2 and CO2/CH4 gas selectivities for HCPANIS-1 mL-160 °C were calculated to be 50.17, 4.39 and 11.41, respectively at 0 °C and 66.05, 7.75 and 8.52, respectively at 25 °C, using the initial slope method (Henry's law constant) (Fig. S16†).5,28 The estimated CO2/N2 selectivity is higher than many of the recently reported nanoporous organic polymers such as, P-1,42 and CPOP-1,53 however, lower than some of the zeolites and hybrid materials.28
53 and are synthesized from expensive precursors than that of EBPANI employed in the present work. HCPANIS-1 mL-120 °C, HCPANIS-1 mL-140 °C, HCPANIS-1 mL-180 °C, HCPANIS-0.5 mL-160 °C, HCPANIS-2 mL-160 °C and HCPANIS-3 mL-160 °C also showed noteworthy CO2 uptake of 9.1 wt%, 9.9 wt%, 10.3 wt%, 9.2 wt%, 13.6 wt% and 6.9 wt% at 0 °C, respectively (Fig. 3b). The CO2 sorption isotherms measured at both 0 °C and 25 °C are almost reversible with minor hysteresis which extends from the high pressure range to the low pressure range (Fig. 3b and S13a†). This indicates that the interaction was neither purely physisorption nor purely chemisorption in nature. This was further confirmed from the isosteric heat of adsorption (Qst) in the range of 27.62 kJ mol−1 to 35.17 kJ mol−1 calculated using Clausius–Clapeyron equation (Fig. S14†). The CH4 uptake of HCPANIS-1 mL-160 °C were observed to be 1.8 wt% and 0.86 wt% at 0 °C and 25 °C, respectively, as shown in Fig. 3c and S13b.† Both the isotherms were completely reversible and the calculated Qst at zero coverage was estimated to be 21.38 kJ mol−1 (Fig. S15†). The details of methane adsorption for other synthesized specimens are shown in Table 2. The CO2/N2, CH4/N2 and CO2/CH4 gas selectivities for HCPANIS-1 mL-160 °C were calculated to be 50.17, 4.39 and 11.41, respectively at 0 °C and 66.05, 7.75 and 8.52, respectively at 25 °C, using the initial slope method (Henry's law constant) (Fig. S16†).5,28 The estimated CO2/N2 selectivity is higher than many of the recently reported nanoporous organic polymers such as, P-1,42 and CPOP-1,53 however, lower than some of the zeolites and hybrid materials.28
| Sample ID | CO2 at 1 bara | CH4 at 1 bara | H2 at 1 bara | |||||
|---|---|---|---|---|---|---|---|---|
| 0 °C | 25 °C | Qst | 0 °C | 25 °C | Qst | −196 °C | Qst | |
| a Gas uptake in wt% and the isosteric heats of adsorption (Qst) in kJ mol−1. | ||||||||
| HCPANIS-1 mL-120 °C | 9.1 | 5.1 | 32.5 | 0.77 | 0.35 | 24.0 | 0.66 | 6.3 |
| HCPANIS-1 mL-140 °C | 9.9 | 5.4 | 28.7 | 1.1 | 0.61 | 17.0 | 0.91 | 5.3 |
| HCPANIS-1 mL-160 °C | 14.7 | 8.7 | 33.6 | 1.8 | 0.86 | 21.4 | 1.18 | 6.2 |
| HCPANIS-1 mL-180 °C | 10.3 | 6.2 | 34.9 | 1.2 | 0.78 | 15.5 | 0.93 | 6.0 |
| HCPANIS-0.5 mL-160 °C | 9.2 | 5.4 | 35.2 | 0.96 | 0.48 | 21.3 | 0.65 | 6.2 |
| HCPANIS-2 mL-160 °C | 13.6 | 8.8 | 33.9 | 0.94 | 0.50 | 20.4 | 1.20 | 7.0 |
| HCPANIS-3 mL-160 °C | 6.9 | 5.3 | 27.6 | 0.90 | 0.54 | 14.4 | 0.62 | 6.6 |
Due to the presence of ultramicropores, HCPANISs were further employed to study the H2 storage capacity at −196 °C and 1 bar (Fig. 3d). It was observed that the maximum H2 storage capacity of 1.20 wt% was obtained for HCPANIS-2 mL-160 °C. This H2 storage capacity could be comparable to the best reported materials. The detailed comparison is given in Table S2.† It is important to note that the adsorption does not show saturation at 1 bar (Fig. 3d) indicating that a higher hydrogen storage capacity can be achieved by applying higher pressure. The complete reversible nature of isotherm as shown in Fig. 3d and the very low value of Qst ≈ 6.2 kJ mol−1 indicates the physisorption of adsorbates (Fig. S17†). Other synthesized specimens HCPANIS-1 mL-120 °C, HCPANIS-1 mL-140 °C, HCPANIS-1 mL-160 °C, HCPANIS-1 mL-180 °C, HCPANIS-0.5 mL-160 °C, and HCPANIS-3 mL-160 °C showed H2 storage capacity of 0.66, 0.91, 1.18, 0.93, 0.65 and 0.62 wt%, respectively.
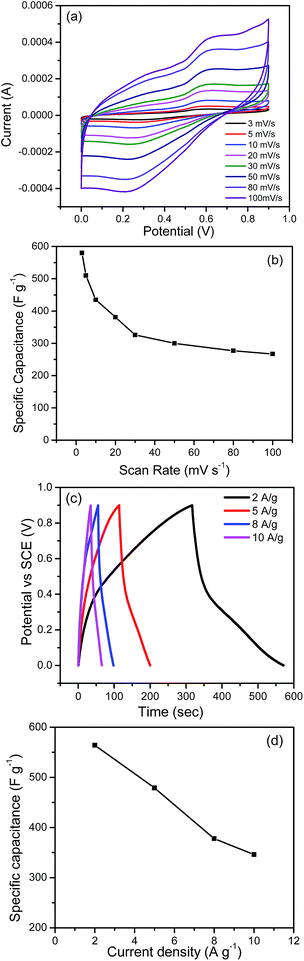
The high surface area and hierarchical porous structure as depicted from N2 sorption isotherm encouraged us to study the electrochemical supercapacitor performance of HCPANIS-1 mL-160 °C. Electrochemical activity of HCPANIS-1 mL-160 °C was studied using CV and GCD. Fig. 4 presents the CVs recorded at different scan rates in 1 M H2SO4 electrolyte in the potential window of 0 V to 0.9 V. An anodic peak is observed at ∼0.6 V during the forward scan suggesting the oxidation of emeraldine base to pernigraniline, which is reduced in the backward cathodic scan. Generally, polyaniline shows two pair of redox peaks in the potential range of 0 to 0.8 V, but in present case one pair of redox peaks is observed. This is due to the PANI used in the present study already exists in emeralidine base form and cross linked via –CH2 linkage to neighbouring PANI chain. For this chemical environment in the PANI chain, only one pair of redox peak is obtained in the cyclic voltammograms. Similar observation has been made in the past with PANI based electrodes.54–56 The presence of redox peaks signifies the pseudocapacitive nature of HCPANIS-1 mL-160 °C electrode. Moreover the response current was found to increase with gradual increase in the scan rate suggesting good electrochemical kinetics of the present electrode. The gravimetric specific electrochemical capacitance (Csp) of electrode material was calculated from following eqn (1).57
 | (1) |
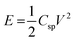 | (2) |
| P = E/t | (3) |
The maximum energy density of 65.25 W h kg−1 was achieved with the expense of a power density of 0.78 kW kg−1. The scan rate dependent energy density and power density behavior of HCPANIS-1 mL-160 °C is presented in Table S3.†
The GCD measurement was carried out to understand the electrocapacitive behavior of HCPANIS-1 mL-160 °C for the supercapacitor application. Unlike the linear triangular trajectory exhibited by electric double layer capacitor, the charge–discharge profile of present electrode (HCPANIS-1 mL-160 °C) shows an asymmetric nature. A closer look on the discharge profile reveals that it consists of two different discharge characteristics over the studied potential window (Fig. 4c). The two discharge regions were distinguished as (i) relatively faster decrease of electrode potential in the range of 0.9 to 0.4 V and (ii) a slower decrease below 0.4 V. The initial fast decrease is attributed to the discharge of electrostatically stored charge on the double layer region of electrode and the second slow decrease in potential corresponds to the discharge of faradically stored charge. The Csp of HCPANIS-1 mL-160 °C was measured from GCD curve using eqn (4).57
 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 5a). The retention of specific capacitance was found to be 68% over the whole charge–discharge test period, suggesting potential of HCPANIS-1 mL-160 °C as electrode material for supercapacitor.
000 cycles (Fig. 5a). The retention of specific capacitance was found to be 68% over the whole charge–discharge test period, suggesting potential of HCPANIS-1 mL-160 °C as electrode material for supercapacitor.
The electrochemical impedance spectroscopy was further employed to obtain insight on the charge transfer behavior of HCPANIS-1 mL-160 °C. Fig. 5b represents the complex impedance plot of HCPANIS-1 mL-160 °C electrode before and after 1000 cycles of charge–discharge. The experimental data was simulated with an equivalent circuit diagram consisting of solution resistance (Rs), charge transfer resistance (Rct), Warburg element (W) and constant phase element (CPE). Both the plot has a semicircle in high frequency region followed by a straight line over medium to low frequency. The diameter of semicircle gives one of the most important impedance characteristics i.e. Rct of the electrode electrolyte interface. The lower the Rct better is the electrode kinetics and thereby improved electrochemical charge storage. The present HCPANIS-1 mL-160 °C electrode exhibits a Rct of 13.43 Ω along with a small Rs of 6.9 Ω. This small Rct is responsible for the easy charge transfer and facile ion diffusion in the porous HCPANIS-1 mL-160 °C electrode resulting in improved electrode kinetics. However a significant increase of Rct was noticed after 1000 charge–discharge cycles. This increase of Rct can be attributed to the intercalation of ionic species during long term cycling process.59 The capacitive characteristics of an electrode can be obtained from CPE element of the Nyquist plot. Generally, CPE is appeared due to the non-uniform current distribution at an inhomogeneous electrode surface. The CPE can be defined as ZCPE = 1/c(jω)n where ‘c’ is the ideal capacitance and ‘n (0 ≤ n ≤ 1)’ is an empirical constant. The magnitude of constant ‘n’ determines the capacitive and resistive characteristics of an electrode where n = 1 represents an ideal capacitive behavior and n = 0 reflects complete resistor type characteristic. In the present study, HCPANIS-1 mL-160 °C electrode shows a ‘n’ value of 0.81 which suggests its good electrocapacitive nature.
Conclusions
In summary, we demonstrate a simple solvothermal method to synthesize HCPANISs for the first time. The as-synthesized HCPANISs show a high specific surface area up to 711 m2 g−1 with ultramicropores. The presence of nitrogen in the HCPANISs frameworks led to a high CO2 uptake whereas the ultra small size pores facilitated the H2 storage capacity. The CO2 uptake and H2 storage per effective specific surface area is found to be higher than several recent reports on organic polymers. Furthermore, the material has shown much higher supercapacitive performance with specific capacitance of Csp = 580 F g−1 at scan rate of 3 mV s−1 with 68% retention capacity over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at current density of 10 A g−1. Thus, the HCPANISs have the potential to be used as the material for clean energy generation and storage.
000 cycles at current density of 10 A g−1. Thus, the HCPANISs have the potential to be used as the material for clean energy generation and storage.
Acknowledgements
The work is supported by DST, Govt. of India [Grant No. DST/IS-STAC/CO2-SR-132/12(G)]. VS acknowledges the CSIR, Govt. of India for the SRF fellowship.References
- A. A. Syed and M. K. Dinesan, Talanta, 1991, 38, 815–837 CrossRef CAS PubMed.
- H. Letheby, J. Chem. Soc., 1862, 15, 161–163 RSC.
- A. G. MacDiarmid, J. C. Chiang, M. Halpern, W. S. Huang, S. L. Mu, L. D. Nanaxakkara, S. W. Wu and S. I. Yaniger, Mol. Cryst. Liq. Cryst., 1985, 121, 173–180 CrossRef CAS.
- H. Zhang, J. Wang, X. Gao, Z. Wang and S. Wang, Synth. Met., 2014, 187, 46–51 CrossRef CAS.
- V. Sharma, A. Sahoo, Y. Sharma and P. Mohanty, RSC Adv., 2015, 5, 45749–45754 RSC.
- W. Chen, R. B. Rakhi and H. N. Alshareef, J. Phys. Chem. C, 2013, 117, 15009–15019 CAS.
- K. Chi, Z. Zhang, J. Xi, Y. Huang, F. Xiao, S. Wang and Y. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 16312–16319 CAS.
- H. Li, J. Wang, Q. Chu, Z. Wang, F. Zhang and S. Wang, J. Power Sources, 2009, 190, 578–586 CrossRef CAS.
- X. Z. Gao, H. J. Liu, F. Cheng and Y. Chen, Chem. Eng. J., 2016, 283, 682–691 CrossRef CAS.
- C. Zhong, C. Duan, F. Huang, H. Wu and Y. Cao, Chem. Mater., 2011, 23, 326–340 CrossRef CAS.
- J. Gong, Y. Li, Z. Hu, Z. Zhou and Y. Deng, J. Phys. Chem. C, 2010, 114, 9970–9974 CAS.
- T. Yang, L. Meng, J. Zhao, X. Wang and K. Jiao, ACS Appl. Mater. Interfaces, 2014, 6, 19050–19056 CAS.
- S. Virji and H. W. Bruce, J. Chem. Educ., 2008, 85, 1102–1104 CrossRef CAS.
- S. K. Shukla, A. Tiwari, G. K. Parashar and G. C. Dubey, Adv. Mater. Lett., 2010, 1, 129–134 CrossRef CAS.
- M. Molberg, D. Crespy, P. Rupper, F. Nüesch, J. A. E. Manson, C. Lowe and D. M. Opris, Adv. Funct. Mater., 2010, 20, 3280–3291 CrossRef CAS.
- J. Germain, J. M. J. Frechet and F. Svec, J. Mater. Chem., 2007, 17, 4989–4997 RSC.
- J. Germain, F. Svec and J. M. J. Frechet, Chem. Mater., 2008, 20, 7069–7076 CrossRef CAS.
- M. Ayad and S. Zaghlol, Chem. Eng. J., 2012, 204–206, 79–86 CrossRef CAS.
- https://www.apps.webofknowledge.com/Search.do?product=UA%26SID=S1ZPtGiFle34FocxjZp%26search_mode=GeneralSearch%26prID=335ca55e–5d85-4188-a9e6-997903edeb6e.
- Y. J. He, Mater. Lett., 2005, 59, 2133–2136 CrossRef CAS.
- J. M. Du, Z. M. Liu, B. X. Han, Z. H. Li and J. L. Zhang, Microporous Mesoporous Mater., 2005, 84, 254–260 CrossRef CAS.
- M. Pang, G. Long, S. Jiang, Y. Ji, W. Han, B. Wang, X. Liu, Y. Xi, D. Wang and F. Xu, Chem. Eng. J., 2015, 280, 377–384 CrossRef CAS.
- D. L. Sivadas, R. Narasimman, R. Rajeev, K. Prabhakarana and K. N. Ninana, J. Mater. Chem. A, 2015, 3, 16213–16221 CAS.
- T. C. Wang, W. Bury, D. A. Gomez-Gualdron, N. A. Vermeulen, J. E. Mondloch, P. Deria, K. Zhang, P. Z. Moghadam, A. A. Sarjeant, R. Q. Snurr, J. F. Stoddart, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2015, 137, 3585–3591 CrossRef CAS PubMed.
- S. Z. Zeng, L. Guo, Q. He, Y. Chen, P. Jiang and J. Shi, Microporous Mesoporous Mater., 2010, 131, 141–147 CrossRef CAS.
- G. Thangamani, K. Deshmukh, M. B. Ahamed, K. Chidambaram, K. C. Saranya and S. K. Pasha, Int. J. ChemTech Res., 2015, 8, 70–76 CAS.
- Y. Zhang, S. Wei, F. Liu, Y. Du, S. Liu, Y. Ji, T. Yokoi, T. Tatsumi and F. S. Xiao, Nano Today, 2009, 4, 135–142 CrossRef CAS.
- P. Mohanty, L. D. Kull and K. Landskron, Nat. Commun., 2011, 2, 401, DOI:10.1038/ncomms1405.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- P. Rekha, U. Sahoo and P. Mohanty, RSC Adv., 2014, 4, 34860–34863 RSC.
- R. Muhammad, P. Rekha and P. Mohanty, RSC Adv., 2016, 6, 17100–17105 RSC.
- P. Rekha, V. Sharma and P. Mohanty, Microporous Mesoporous Mater., 2016, 219, 93–102 CrossRef CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- M. Zhi, C. Xiang, J. Li, M. Li and N. Wu, Nanoscale, 2013, 5, 72–88 RSC.
- W. Y. Zheng and K. Levon, Macromolecules, 1994, 27, 7754–7768 CrossRef CAS.
- B. Stuart, Infrared Spectroscopy: Fundamentals and Applications, John Wiley & Sons, 2004, p. 80 Search PubMed.
- C. D. Wood, B. Tan, A. Trewin, H. Niu, D. Bradshaw, M. J. Rosseinsky, Y. Z. Khimyak, N. L. Campbell, R. Kirk, E. Stockel and A. I. Cooper, Chem. Mater., 2007, 19, 2034–2048 CrossRef CAS.
- S. Kaplan, E. M. Conwell, A. F. Richter and A. G. MacDiarmid, J. Am. Chem. Soc., 1988, 110, 7647–7651 CrossRef CAS.
- P. M. Budd, A. Butler, J. Selbie, K. Mahmood, N. B. McKeown, B. Ghanem, K. Msayib, D. Book and A. Walton, Phys. Chem. Chem. Phys., 2007, 9, 1802–1808 RSC.
- H. A. Patel, S. H. Je, J. Park, D. P. Chen, Y. Jung, C. T. Yavuz and A. Coskun, Nat. Commun., 2013, 4, 1357, DOI:10.1038/ncomms2359.
- J. Lu and J. Zhang, J. Mater. Chem. A, 2014, 2, 13831–13834 CAS.
- S. Qiao, Z. Du and R. Yang, J. Mater. Chem. A, 2014, 2, 1877–1885 CAS.
- H. A. Patel, D. Ko and C. T. Yavuz, Chem. Mater., 2014, 26, 6729–6733 CrossRef CAS.
- T. Islamoglu, M. G. Rabbani and H. M. El-Kaderi, J. Mater. Chem. A, 2013, 1, 10259–10266 CAS.
- P. Arab, A. Verlander and H. M. El-Kaderi, J. Phys. Chem. C, 2015, 119, 8174–8182 CAS.
- B. Li, Z. Guan, X. Yang, W. D. Wang, W. Wang, I. Hussain, K. Song, B. Tan and T. Li, J. Mater. Chem. A, 2014, 2, 11930–11939 CAS.
- B. Zhang, G. Li, J. Yan and Z. Wang, J. Phys. Chem. C, 2015, 119, 13080–13087 CAS.
- M. G. Rabbani and H. M. El-Kaderi, Chem. Mater., 2011, 23, 1650–1653 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974–986 CrossRef CAS PubMed.
- N. P. Wickramaratne and M. Jaroniec, ACS Appl. Mater. Interfaces, 2013, 5, 1849–1855 CAS.
- Y. Zhu, H. Long and W. Zhang, Chem. Mater., 2013, 25, 1630–1635 CrossRef CAS.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed.
- Q. Chen, M. Luo, P. Hammershoj, D. Zhou, Y. Han, B. W. Laursen, C. G. Yan and B. H. Han, J. Am. Chem. Soc., 2012, 134, 6084–6087 CrossRef CAS PubMed.
- S. Cho, O. S. Kwon, S. A. You and J. Jang, J. Mater. Chem. A, 2013, 1, 5679–5688 CAS.
- X. Wang, J. Deng, X. Duan, D. Liu, J. Guo and P. Liu, J. Mater. Chem. A, 2014, 2, 12323–12329 CAS.
- X. Li, L. Yang, Y. Lei, L. Gu and D. Xiao, ACS Appl. Mater. Interfaces, 2014, 6, 19978–19989 CAS.
- V. Sahu, S. Shekhar, R. K. Sharma and G. Singh, ACS Appl. Mater. Interfaces, 2015, 7, 3110–3116 CAS.
- V. Gupta and N. Miura, Mater. Lett., 2006, 60, 1466–1469 CrossRef CAS.
- B. Wang, Q. Liu, Z. Qian, X. Zhang, J. Wang, Z. Li, H. Yan, Z. Gao, F. Zhao and L. Liu, J. Power Sources, 2014, 246, 747–753 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Reaction scheme, FTIR spectra and XRD patterns of HCPANISs, TGA/DTG thermogram of HCPANIS-1 mL-160 °C, multipoint BET and Langmuir plots of HCPANISs, pore size distribution of HCPANIS-1 mL-160 °C, CO2 and CH4 adsorption isotherms of HCPANISs measured at 25 °C, comparative study of CO2 and H2 storage, isosteric heats of adsorption of CO2, CH4 and H2 adsorption, gas selectivity plots of HCPANIS-1 mL-160 °C measured at 0 °C and 25 °C. See DOI: 10.1039/c6ra06252k |
| This journal is © The Royal Society of Chemistry 2016 |