Influence of the aromatic moiety in α- and β-arylalanines on their biotransformation with phenylalanine 2,3-aminomutase from Pantoea agglomerans†‡
Abstract
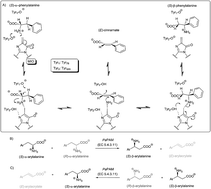
In this study enantiomer selective isomerization of various racemic α- and β-arylalanines catalysed by phenylalanine 2,3-aminomutase from Pantoea agglomerans (PaPAM) was investigated. Both α- and β-arylalanines were accepted as substrates when the aryl moiety was relatively small, like phenyl, 2-, 3-, 4-fluorophenyl or thiophen-2-yl. While 2-substituted α-phenylalanines bearing bulky electron withdrawing substituents did not react, the corresponding substituted β-aryl analogues were converted rapidly. Conversion of 3- and 4-substituted α-arylalanines happened smoothly, while conversion of the corresponding β-arylalanines was poor or non-existent. In the range of pH 7–9 there was no significant influence on the conversion of racemic α- or β-(thiophen-2-yl)alanines, whereas increasing the concentration of ammonia (ammonium carbonate from 50 to 1000 mM) inhibited the isomerization progressively and decreased the amount of the by-product (i.e. (E)-3-(thiophen-2-yl)acrylic acid was detected). In all cases, the high ee values of the products indicated excellent enantiomer selectivity and stereospecificity of the isomerization except for (S)-2-nitro-α-phenylalanine (ee 92%) from the β-isomer. Substituent effects were rationalized by computational modelling revealing that one of the main factors controlling biocatalytic activity was the energy difference between the covalent regioisomeric enzyme–substrate complexes.

 Please wait while we load your content...
Please wait while we load your content...