Freezing-mediated polymerization of Ag nanoparticle-embedded polyaniline belts with polyoxometalate as doping acid exhibiting UV-photosensitivity†
Abstract
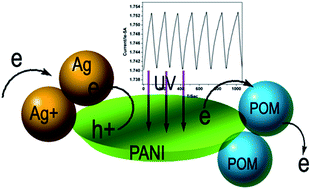
Ag nanoparticle-embedded polyaniline belts have been synthesized via the freezing polymerization of aniline assisted by polyoxometalate and silver nitrate. The material exhibits a significant UV photoresponse, which can be attributed to the efficient dissociation of the photo-induced hole trapped by dispersed silver nanoparticles and the photo-induced electron exported by the polyoxometalate.

 Please wait while we load your content...
Please wait while we load your content...