Advanced generation of paeonol-phenylsufonyl derivatives as potential anti-HBV agents†
Y. P. Huangai,
H. P. Shiha,
Y. C. Liangb,
H. H. Linc,
M. C. Lind,
C. W. Chenef,
T. J. Huang*g,
Y. C. Kuo*h,
C. C. Han*a and
M. H. Hsu*ij
aDepartment of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan
bAgricultural Biotechnology Research Center, Academia Sinica, Taipei 115, Taiwan
cDivision of Radiotherapy, Department of Oncology, Taipei Veterans General Hospital, Taipei 115, Taiwan
dBiomedical Technology and Device Research Laboratories, Industrial Technology Research Institute, Hsinchu 30013, Taiwan
eDepartment of Anesthesiology, China Medical University Hospital, Taichung 404, Taiwan
fDepartment of Anesthesiology, School of Medicine, China Medical University, Taichung 404, Taiwan
gSchool of Medicine, China Medical University, Taichung 404, Taiwan
hRadiation Oncology, Show Chwan Memorial Hospital, Changhua City 50008, Taiwan
iNuclear Science & Technology Department Center, National Tsing Hua University, Hsinchu 30013, Taiwan. E-mail: mhhsu@mx.nthu.edu.tw; Fax: +886-3-572-5974; Tel: +886-3-573-1180
jDepartment of Chemistry, Tamkang University, New Taipei City 25137, Taiwan
First published on 18th April 2016
Abstract
Hepatitis B virus (HBV) infection causes serious liver diseases, and the development of effective drugs for chronic hepatitis B treatment remains an important step towards the eradication of HBV worldwide. Recently, our group designed paeonol-phenylsulfonyl derivatives and found that the compound 2-acetyl-5-methoxyphenyl 4-methoxybenzenesulfonate (6f) had the most potent antiviral effect against HBV, with an IC50 value of 0.36 μM and a high selectivity index (SI; TC50/IC50) of 47.75. In this research, we modified compound 6f with a 2-aminothiazole scaffold and generated 13 novel paeonol derivatives by utilizing various benzoyl and acyl chlorides. Among this new generation, compound 2-(2-benzamidothiazol-4-yl)-5-methoxyphenyl 4-methoxybenzenesulfonate (8a) showed the highest SI value of 59.14 which exceeds those of compound 6f and lamivudine (3TC), a commercially available antiviral drug. Hence, we believe that our studies may offer some useful information for the development of antiviral medicines.
1. Introduction
Hepatitis B virus (HBV), a member of the Hepadnaviridae family, is a causative agent in progressive liver diseases. Nowadays, over two billion people are infected with HBV worldwide and approximately 400 million are chronically infected carriers.1,2 Moreover, 80% of chronic HBV carriers have varying degrees of liver damage, which could progress to liver cirrhosis and hepatocellular carcinoma.3 At present, the clinically available anti-HBV agents are all nucleoside or nucleotide analogs that target the activity of viral reverse transcriptase (RT), which regulates minus strand DNA synthesis, the first step in viral genome replication from the pregenomic RNA template;4 however, these anti-HBV drugs are reported to have viral resistance owing to specific RT mutations. Thus, it is important to develop new therapeutic agents that adopt other approaches to ameliorate the disease.Many compounds isolated from botanical resources are alleged to have antiviral activity toward HBV.5–7 In our previous study,8 we derivatized paeonol (4; Scheme 1), the main active component of a traditional Chinese herbal medicine Moutan Cortex that has anti-inflammatory,9,10 analgesic,10 and antiatherogenic11 activity, with various phenylsulfonyl groups (Scheme 1). The results demonstrated that the compound 2-acetyl-5-methoxyphenyl 4-methoxybenzenesulfonate (6f; Scheme 1) had the most potent inhibitory effect on viral gene expression and viral propagation in a cell culture model, with an IC50 value of 0.36 μM and a high selectivity index (SI; TC50/IC50) of 47.75. We then modified 6f with a 2-aminothiazole scaffold (7; Scheme 2), a moiety contained in a number of commercially available drugs such as abafungin (1; Fig. 1), an antimicrobial agent, meloxicam (2; Fig. 1), a nonsteroidal anti-inflammatory drug used in arthritis, dysmenorrhea and fever, and talipexole (3; Fig. 1), which is used as an anti-Parkinson molecule.12 Caravatti et al. presented a potent and selective phosphatidylinositol-3 kinase alpha inhibitor which bore this moiety and had therapeutic potential for treating cancers.13 Costantino et al. reported N-substituted 2-aminothiazole derivatives with inhibitory activity toward Mycobacterium tuberculosis H37Rv.14 From the perspective of medicinal chemistry, the 2-aminothiazole moiety may serve as a stable bioisostere of a phenol group.15 Like the phenol group, such a structure also has antioxidant properties; in addition, compared to the phenol group, the 2-aminothiazole scaffold is more lipophilic and displays improved oral availability. In this study, we developed a 2-aminothiazole core embodying paeonol derivatives with a 4-methoxyphenylsulfonyl side chain on its phenol group, and further substituted the amino group with para-position substituted benzoyl chlorides and acyl chlorides. The anti-HBV effects of these novel compounds in HepG2 2.2.15 cells were evaluated and the results are described in the following text.
2. Experimental section
2.1. Chemistry
All reactions were carried out in oven-dried glassware (120 °C) under an atmosphere of nitrogen. Acetone, dichloromethane, ethyl acetate and hexane from Mallinckrodt Chemical Co. were dried and distilled from CaH2. Paeonol, 4-methoxybenzenesulfonyl chloride, potassium carbonate, thiourea, iodine, sodium hydroxide, benzoyl chloride, 4-fluorobenzoyl chloride, 4-chlorobenzoyl chloride, 4-bromobenzoyl chloride, 4-toluoylbenzoyl chloride, 4-(trifluoromethyl)benzoyl chloride, 4-methoxybenzoyl chloride, 4-nitrobenzoyl chloride, acetyl chloride, propionyl chloride, and butyryl chloride were purchased from Sigma-Aldrich Chemical Co without further purification. Analytical thin layer chromatography (TLC) was performed on precoated plates (silica gel 60 F-254), purchased from Merck Inc. Purification by column chromatography was carried out with Merck Reagents Silica Gel 60 (particle size 0.063–0.200 mm, 70–230 mesh ASTM).Proton NMR spectra were obtained on a Bruker Avance 500 (500 MHz) and a Varian Unity-400 (400 MHz) with d-chloroform and d-acetone as solvent. Proton NMR chemical shifts are referenced to the CDCl3 singlet (7.24 ppm) and the center of (CD3)2CO quintet (2.05 ppm). Carbon-13 NMR spectra were obtained on a Varian UNITY INOVA 500 (125 MHz) by use of chloroform-d and d-acetone as solvent. Carbon-13 chemical shifts are referenced to the center of the CDCl3 triplet (77.0 ppm) and (CD3)2CO heptet (29.85 ppm). Multiplicities are recorded by the following abbreviations: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; J, coupling constant (Hertz). High-resolution mass spectra were obtained with a VARIAN 901-MS (FT-ICR Mass) mass spectrometer. High-performance liquid chromatography (HPLC) analyses were carried out by Agilent 1100 series system with CNW Athena C18 column (120 Å, 4.6 mm × 250 mm, 5 μm) and UV detection at 254 nm.
2.2. Biology
3. Results and discussion
3.1. Synthesis
To obtain various 2-aminothiazole core-modified paeonol derivatives with 4-methoxyphynylsulfonyl side chains on the phenol group, we first treated paeonol with 4-methoxyphenylsulfonyl chloride to yield 6f (Scheme 1). Then, 6f was reacted with thiourea and iodine in ethanol under reflux conditions, which afforded compound 7 (Scheme 2). Amino groups on thiazole rings were then processed through nucleophilic substitutions with 4-position substituted benzoyl chlorides and aliphatic acyl chlorides to generate the desired compounds (Scheme 2). All the products were purified to more than 95% by column chromatography for bioassays.3.2. Cytotoxic effect of the compounds on HepG2 2.2.15 hepatoma cells
To understand the structure–activity relationship (SAR) and determine the cytotoxic effect of compounds 8a–9e on HepG2 2.2.15 cells, the cells were subjected to treatment with each compound which was diluted in a two-fold serial order, for 72 h, and the viability of the cells was measured according to the manufacturer's protocol. All measurements were performed four times and results were presented as a relative percentage of those of the control group. In Table 1, according to TC50 values, compound 8a, 8e, 8f, 8g and 8h are less cytotoxic when compared to 5-FU, and substituting H atom at para positions on benzoyl rings with halogen atoms F, Cl and Br, which correspond to compounds 8b, 8c and 8d, increases the cytotoxicity. To our surprise, replacing the aromatic ring with more flexible aliphatic alkyl chains, which corresponds to compounds 9a–9e, significantly enhances the cytotoxic effect. Additionally, the activity of compound 8b bearing F, the bioisostere of H, is similar to that of compound 8a. Another two bioisostere sets, Cl and Br on compounds 8c and 8d and CH3 and CF3 on compounds 8e and 8f, show the same phenomenon as those of 8a and 8b. Compared with compounds 8a–8d, activities of compounds 8f–8h are much lower, and this may result from relatively larger steric hindrances caused by the 4-position substituting groups on benzene rings of 8f–8h. These results suggest that the 4-position on the benzoyl ring may play a crucial role in the anti-HBV actions of these compounds. In addition, displacing a relatively rigid aromatic moiety with a more flexible aliphatic alkyl chain on the side chain of amide group may, however, endow the compound a much higher cytotoxicity thus lowering its SI value drastically.| Compound | TC50 (μM) | HBsAg IC50 (μM) | HBeAg IC50 (μM) | Inhibition of HBV DNA replication IC50 (μM) | HBV DNA replication SI value (TC50/IC50) |
|---|---|---|---|---|---|
| a TC50: the concentration of the compound at which cell viability was reduced to 50%. IC50: the concentration of the compound at which anti-HBV effect was reached to 50%. *5-FU: fluorouracil, the positive control for cytotoxic analysis; the structure is shown in Fig. S2 of the ESI. *Lamivudine (3TC), the positive control for anti-HBV analysis; the structure is shown in Fig. S2 of the ESI. SI: selectivity index; TC50/IC50. | |||||
| 8a | 373.99 ± 5.52 | 3.30 ± 0.32 | 4.27 ± 0.32 | 6.32 ± 0.31 | 59.14 ± 3.51 |
| 8b | 145.47 ± 3.45 | 3.96 ± 0.12 | 8.34 ± 1.34 | 12.67 ± 1.21 | 11.48 ± 1.32 |
| 8c | 144.80 ± 4.21 | 5.52 ± 0.6 | 5.48 ± 0.45 | 7.25 ± 1.41 | 19.97 ± 4.72 |
| 8d | 204.80 ± 3.36 | 8.13 ± 0.24 | 19.88 ± 2.12 | 17.78 ± 2.87 | 11.52 ± 2.22 |
| 8e | 508.32 ± 3.54 | 107.09 ± 2.25 | 173.41 ± 3.42 | 153.26 ± 3.65 | 3.32 ± 0.1 |
| 8f | 416.93 ± 4.32 | 86.21 ± 1.64 | 116.87 ± 4.5 | 99.81 ± 1.78 | 4.18 ± 0.1 |
| 8g | 479.78 ± 4.65 | 85.99 ± 2.57 | 173.76 ± 3.74 | 161.65 ± 4.43 | 2.97 ± 0.1 |
| 8h | 581.53 ± 4.45 | 83.76 ± 3.68 | 96.52 ± 3.21 | 84.24 ± 3.12 | 6.90 ± 0.3 |
| 9a | 54.29 ± 2.33 | 6.22 ± 0.12 | 7.63 ± 1.36 | 5.89 ± 0.21 | 9.22 ± 0.59 |
| 9b | 12.89 ± 2.24 | 2.85 ± 0.25 | 4.02 ± 0.64 | 4.00 ± 0.87 | 3.22 ± 1.1 |
| 9c | 47.20 ± 2.67 | 3.67 ± 0.64 | 4.13 ± 1.41 | 3.44 ± 0.11 | 13.71 ± 1.03 |
| 9d | 13.02 ± 1.21 | N/A | N/A | N/A | N/A |
| 9e | 64.98 ± 3.47 | 5.73 ± 0.22 | 11.36 ± 2.21 | 13.99 ± 0.84 | 4.65 ± 0.43 |
| 5-FU* | 216.79 ± 5.74 | — | — | — | — |
| Lamivudine (3TC)* | 352.03 ± 4.38 | — | — | 7.63 ± 0.41 | 46.13 ± 2.95 |
3.3. Antiviral effect of compounds on HBV viral antigen expression in HepG2 2.2.15 cell culture medium
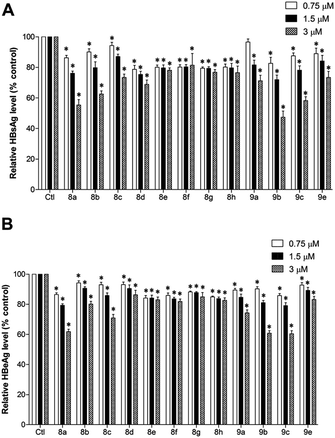
Fig. 2 demonstrates the inhibition effect of compounds 8a–9e on HBV viral antigen expression and secretion in HepG2 2.2.15 cell culture media. All compounds could effectively reduce secretion of HBsAg and HBeAg at low doses, and compound 8a had the most potent inhibitory effect. As concentrations of compounds 8a–d and 9a–e increased, the expression of HBsAg and HBeAg decreased; on the other hand, the results did not show pronounced changes when cells were treated with compounds 8e–8h. Such phenomena may be attributed to the relatively large steric hindrance that the 4-position substituting groups cast on the benzene rings of compounds 8e–8h.To further confirm the effect of 8a on HepG2 2.2.15 proliferation, we assessed the morphology of growth response of cells employing the Giemsa staining method. The general cellular morphology after 8a treatment is shown in Fig. 3A. There was no significant difference in the morphology of HepG2 2.2.15 cells in the groups treated with compound 8a and the control group (Fig. 3A). To examine the role of compound 8a in HepG2 2.2.15 cell cycle progression, we performed flow cytometry analysis. DNA of the HepG2 2.2.15 cells were stained with PI to analyze the population of DNA content as index of cell cycle after treatment with 8a (Fig. 3B). The ratio of cells in each phase of the cell cycle did not decline as the dose of 8a rose. These results suggest that 8a did not affect the HepG2 2.2.15 cell growth or cell cycle in treated dose range that have inhibitory effect on HBV.
3.4. Antiviral effect of compounds on HBV DNA replication in HepG2 2.2.15 cells
To investigate the antiviral activity of compounds in HBV DNA replication, HepG2 2.2.15 cells were treated with three non-cytotoxic concentrations of each compound for 48 h. The cultural media were harvested and the viral genomic DNA of the secreted virion particles was isolated using real-time PCR analysis. The results, shown in Fig. 4 reveal that compounds 8a–9e could reduce the replication amounts of viral DNA at low doses, and in comparison with lamivudine (3TC), compounds 8a had apparent inhibitory effects on viral DNA replication whereas 8e–8h did not. Fig. 5 illustrates the selectivity index (SI; TC50/IC50) values of each compound on viral DNA replication, and indicates that the SI value of compound 8a is the highest of all the novel compounds and even better than those of the commercially available antiviral drug 3TC and our previously synthesized compound 6f.3.5. Effect of compound 8a on HBV viral gene expression
We further examined the antiviral effect of 8a, which showed potent inhibition of HBV viral gene expression. HepG2 2.2.15 cells were treated with three non-cytotoxic concentrations of compound 8a for 48 h, and the total cellular RNA was extracted and subjected to northern blot analysis of HBV viral RNA levels. As shown in Fig. 6, treatment of 8a significantly lowered the levels of 3.5 kb precore/pregenomic and 2.4-/2.1 kb surface antigen RNA in a dose-dependent manner.4. Conclusion
Based on our previous study, we further optimized the 4-methoxyphenylsulfonyl modified paeonol with a 2-aminothiazole core and generated 13 novel paeonol derivatives by utilizing various benzoyl and acyl chlorides. All the compounds were characterized and their anti-HBV activities were assessed. We conducted cell viability assessment, cell cycle analysis, and dose-dependent inhibition experiments on viral DNA replication and HBV gene and viral antigen expression to address the SAR and mechanism issues. The results showed that compound 8a had potent activity against HBV in HepG2 2.2.15 cells with IC50 value of 6.32 μM and a remarkable selectivity index (TC50/IC50) of 59.14 that surpassed those of our previously synthesized compound 6f and 3TC, a commercially available antiviral nucleoside medicine. The compound may be a promising lead for anti-HBV therapy.Acknowledgements
For financial support, we thank the Ministry of Science and Technology of the Republic of China, National Tsing Hua University, China Medical University Hospital, and Show Chwan Memorial Hospital. This work was supported by the Ministry of Science and Technology of Taiwan (Grant 104-2119-M-007-017-) (Grant 105-2623-E-007-009-), China Medical University Hospital (DMR-103-042) (DMR-103-071) (DMR-105-044), and Show Chwan Memorial Hospital Research Foundation (RD105020). The authors express heartfelt thanks to Dr Pei-Jer Chen for HepG2 2.2.15 cells as a model for antiviral research, the Laboratory Animal Core Facility which is funded by the Agricultural Biotechnology Research Center (ABRC) at Academia Sinica for technical support in cell morphology and flow cytometry experiments, and Ms Miranda Jane Loney (ABRC, Academia Sinica, Taiwan) for her critical editing of this manuscript.References
- B. J. McMahon, Semin. Liver Dis., 2005, 25(1), 3–8 CrossRef PubMed.
- H. Popper, D. A. Shafritz and J. H. Hoofnagle, Hepatology, 1987, 7, 764–772 CrossRef CAS PubMed.
- N. H. Park, I. H. Song and Y. H. Chung, Postgrad. Med. J., 2006, 82, 507–515 CrossRef CAS PubMed.
- K.-H. Kim, N. D. Kim and B.-L. Seong, Molecules, 2010, 15, 5878–5908 CrossRef CAS PubMed.
- T.-J. Huang, B.-H. Chou, C.-W. Lin, J.-H. When, C.-H. Chou, L.-M. Yang and S.-J. Lin, Phytochemistry, 2014, 99, 107–114 CrossRef CAS PubMed.
- T.-J. Huang, S.-H. Liu, Y.-C. Kuo, C.-W. Chen and S.-C. Chou, Antiviral Res., 2014, 101, 97–104 CrossRef CAS PubMed.
- L.-P. Qiu and K.-P. Chen, Fitoterapia, 2013, 84, 140–157 CrossRef CAS PubMed.
- T.-J. Huang, H. Chuang, Y.-C. Liang, H.-H. Lin, J.-C. Horng, Y.-C. Kuo, C.-W. Chen, F.-Y. Tsai, S.-C. Yen, S.-C. Chou and M.-H. Hsu, Eur. J. Med. Chem., 2015, 90, 428–435 CrossRef CAS PubMed.
- P.-K. Fu, C.-L. Wu, T.-H. Tsai and C.-L. Hsieh, J. Evidence-Based Complementary Altern. Med., 2012, 2012, 837513 Search PubMed.
- T.-C. Chou, J. Pharmacol., 2003, 139, 1146–1152 CAS.
- K.-C. Pao, J.-F. Zhao, T.-S. Lee, Y.-P. Huang, C.-C. Han, S. L.-C. Huang, K.-H. Wu and M.-H. Hsu, RSC Adv., 2015, 5, 5652–5656 RSC.
- R. J. Nevagi, Pharm. Lett., 2014, 6, 134–150 Search PubMed.
- P. Furet, V. Guagnano, R. A. Fairhurst, P. Imbach-Weese, I. Bruce, M. Knapp, C. Fritsch, F. Blasco, J. Blanz, R. Aichholz, J. Hamon, D. Fabbro and G. Caravatti, Bioorg. Med. Chem. Lett., 2013, 23, 3741–3748 CrossRef CAS PubMed.
- M. Pieroni, B. Wan, S. Cho, S. G. Franzblau and G. Costantino, Eur. J. Med. Chem., 2014, 72, 26–34 CrossRef CAS PubMed.
- L. A. van Vliet, N. Rodenhuis, H. Wikström, T. A. Pugsley, K. A. Serpa, L. T. Meltzer, T. G. Heffner, L. D. Wise, M. E. Lajiness, R. M. Huff, K. Svensson, G. R. M. M. Haenen and A. Bast, J. Med. Chem., 2000, 43, 3549–3557 CrossRef CAS PubMed.
- M. A. Sells, M. L. Chen and G. Acs, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 1005–1009 CrossRef CAS.
- Y. Feng, F. He, P. Zhang, Q. Wu, N. Huang, H. Tang, X. Kong, Y. Li, J. Lu, Q. Chen and B. Wang, Antiviral Res., 2009, 81, 277–282 CrossRef CAS PubMed.
- W. Zou, X. Yang, L. P. Yang, L. Y. Lai, J. H. Lei, H. Y. Luo and Y. H. Zhang, Zhonghua Ganzangbing Zazhi, 2004, 12, 444 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06119b |
| This journal is © The Royal Society of Chemistry 2016 |