Open Access Article
Open Access ArticleFlavonol-based fluorescent indicator for determination of β-glucosidase activity†
Illia E.
Serdiuk
*ab,
Milena
Reszka
a,
Henryk
Myszka
a,
Karol
Krzymiński
a,
Beata
Liberek
a and
Alexander D.
Roshal
b
aDepartment of Chemistry, University of Gdańsk, 80-308 Gdańsk, Poland. E-mail: illia.serdiuk@gmail.com
bInstitute of Chemistry, V. N. Karazin Kharkiv National University, Kharkiv, 61022 Ukraine
First published on 25th April 2016
Abstract
A novel fluorescent probe, based on the excited-state intramolecular proton transfer (ESIPT) phenomenon, for evaluation of β-glucosidase activity was designed. The synthesis of the probe was optimized. The conditions for the monitoring of enzymatic cleavage of the probe were developed and discussed from the point of view of reaction kinetics and simplicity of the method.
Enzymes represent a group of organic macromolecules acting as catalysts for the vital processes occurring in cells.1 In healthy organisms, concentration and activity of enzymes are maintained within strictly defined ranges. Inappropriate activity and changes in structure of some enzymes most often indicate serious dysfunction of a cell or whole organism including genetic disorders in humans like glucose-6-phosphate dehydrogenase (G6PD) deficiency,2 Tay–Sachs3 and Gaucher4 diseases. In the view of indisputable importance of enzymes for living organisms, investigations aimed at developing the new methods of measuring enzymes activity are of high cognitive importance and applicative value.
β-Glucosidases are a group of glycosyl hydrolases which catalyze cleavage of β-glucosidic bonds in disaccharides and other glucose-containing molecules. These enzymes are abundant in microorganisms,5–7 plants,8–10 mammals and humans,11,12 where they play important roles in a wide sort of biological processes. Since action of β-glucosidases leads to release of biologically active molecules from respective glycosides, β-glucosidases have been investigated and employed in different fields of science, medicine and technology. For example, monitoring of the β-glucosidase activity in humans is used in diagnosis of Gaucher disease, which is a genetic disorder characterized by the deficiency of acid β-glucosidase.4,13–15 From the point of view of perspective usage in medicine, the soybean extracts containing isoflavone glucosides cultured with β-glucosidase-producing G. lucidum mycelia exhibit promising features. Isoflavone aglycon which is produced in the result of enzymatic cleavage and is present in these extracts inhibits angiogenesis caused by colon carcinoma cells in mices.16 In botanical studies, an increase of β-glucosidase activity was proved to induce the defense mechanism against F. oxysporum f. sp. lupine and inhibition of pathogen spread.17 In the environmental studies, it was demonstrated that the highest β-glucosidase activity is associated with the phytoplankton bloom breakdown in the eutrophic lake during the spring algal bloom.18 From a biotechnological point of view, it is extremely important to control β-glucosidase activity during the production of second and third generation of biofuels from the renewable lignocellulosic biomass sugars.19 In agriculture, it was reported that evaluation of the β-glucosidase activity in soil can be used as an indicator of its quality.20,21
β-Glucosides of various derivatives of phenol (nitrophenols,22 7-hydroxycoumarin,7 fluorescein,21etc.) are used for estimation of activity of β-glucosidases. Among them, those which can be detected by fluorescence seem to be the most sensitive reagents due to lower detection limits of fluorescent spectroscopy as compared to other spectroscopic methods.23 One of the most popular substrate widely used for estimation of activity of various β-glucosidases seems to be 4-methylumbelliferyl β-D-glucopyranoside (MUG).7,11,18,24 Interaction of MUG with β-glucosidase produces 4-methylumbelliferone (7-hydroxy-4-methylcoumarin), whose anionic form exhibits blue fluorescence at 448 nm.25 Specific enzymatic activity of a tested sample corresponds thus to fluorescence intensity, measured after a certain period of time. As a fluorophore MUG has, however, two drawbacks. Firstly, its blue fluorescence overlaps with the background of biological samples, which requires isolation and purification of enzymes prior estimation of their activity, and complicates the analysis of β-glucosidases of low-activity. Secondly, pKa value of the protolytic equilibrium between neutral and anionic species of 4-methylumbelliferone is near 7.3,25 which indicates that only half of the amount of dye exists in the anionic form at the physiological pH and thus can be utilized in analyses. Fluorescein derivatives, such as di-β-D-glucopyranoside (FDG) also utilized for the purposes discussed,21 in the course of the enzymatic cleavage are transformed to fluorescein, whose anionic species are highly fluorescent, but susceptible to self-reabsorption and other nonradiative deactivation effects under increase of concentration.23 For these reasons, the fluorescence intensity of such dyes shows nonlinear dependence on concentration at above ca. 10−5 M which complicates use of these probes for accurate measurements at higher concentrations.
In an attempt to overcome these problems we have focused our attention on fluorophores in which the excited state intramolecular proton transfer (ESIPT) can occur – particularly the group of flavonols (3-hydroxy-2-phenylchromen-4H-ones). The fluorescence, characterizing such compounds, is distinguished by a substantial red-shifted emission with abnormally high Stokes shift and low susceptibility to self-reabsorption effect,26,27 which enables linear dependence of fluorescence intensity on concentration in a wider range than in the case of common fluorophores like 4-methylumbelliferone and fluorescein. Due to the latter features, use of flavonols and ESIPT fluorophores in general allows to avoid some problems connected with the concentration restrictions and scattering of light in solutions of biological samples with increased turbidity, which provides more possibilities for biological and medical analyses. The attractive spectral features of various derivatives of flavonol mentioned above have been already successfully utilized for investigations of the properties of biological macromolecules,27,28 as well as in the role of enzyme sensors.29 Among the ESIPT fluorophores flavonols represent a group of compounds, which are abundant in plants and make an essential part of human diet.30 Most often flavonols are found in nature and absorbed into digestive system as glucosides,31 which inspired us to create and testify a fluorescent enzyme sensor utilizing ESIPT phenomenon on the basis of flavonol glucoside.
The key idea, realized in this work, is introduction of a substituted flavonol β-glucoside (1) to interaction with β-glucosidase (Scheme 1). Cleavage of the β-glucosidic bond in 1 leads to flavonol derivative (2), whose electronically excited species N* undergo ESIPT to produce tautomeric form denoted as T*. The course of enzymatic cleavage can be thus monitored by the rise of red-shifted fluorescence of T*, centered around 530 nm. Spectral features of 4′-fluoroflavonol (2) are very similar to those of flavonol.32 Besides, susceptibility of fluorine in flavone derivatives to substitution by nucleophiles33 seems to be useful from the point of view of synthesis of other derivatives of flavonol glucosides. For these reasons, 1 was selected for the preliminary investigations aimed to find sensitive ESIPT fluorescent probe on β-glucosidase due to relevant fluorescence properties of 2 and possibility of further chemical modifications of 1.
Two approaches were applied to synthesise 4′-fluoroflavonol β-D-glucopyranoside (1). Both of them utilized the procedure described for glycosylation of other flavonoids.34 Glycosylation of 2 was carried out using 2,3,4,6-tetra-O-acetyl-α-D-glucosyl bromide (4) in a two-phase system CHCl3/H2O for 70 h in the presence of K2CO3 and tetrabutylammonium bromide as a phase-transfer catalyst (Scheme 2). In this way 4′-fluoroflavonol 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside (3) was obtained in 60% yield after column chromatography. Next, 3 was deprotected using sodium methanolate (RT, 1 h) to give 1 in 63% yield. The above described procedure was optimized in such a way, that the product 3 was subjected to deacylation step with K2CO3 in methanol at RT for 10 h without purification to afford the target compound 1 in a satisfactory overall yield of 39% (Scheme 2).
 | ||
| Scheme 2 Synthesis of 1. Conditions: A – K2CO3, Bu4N+Br− in CHCl3/H2O (1/1), 70 h, RT; B – MeONa in MeOH, 1 h, RT; C – K2CO3 in MeOH, 10 h, RT. | ||
The reactivity of the probe 1 in terms of the enzymatic cleavage was assessed using commercially available β-glucosidase from almonds (Sigma-Aldrich). For adequate interpretation of the experimental findings, kinetics of reaction was investigated. Initially, mixture of 1 (5 × 10−5 M) and β-glucosidase (5 × 10−3 units per l) in phosphate buffer saline (PBS, pH = 7.4) was incubated at 37 °C and fluorescence spectra were measured in specified time intervals. However, the changes observed in fluorescence spectra were of unsatisfactory magnitude (spectra not shown), due to low quantum yield of fluorescence of 2 (1.9% in PBS, pH = 7.4, excitation wavelength (λex) 350 nm). Low intensity of fluorescence in water and other protic solvents is a common feature of ESIPT fluorophores. In the group of flavonols this phenomenon is assumed to be caused by disruption of the intramolecular hydrogen bond due to interaction with solvent molecules.35 To overcome the above mentioned problem, we have proposed two solutions. From one perspective, to increase resolution of the fluorescence signal expressed by 2, one should change its environment from protic to aprotic, where its quantum yield of fluorescence (φfl) is substantially higher. Guided by these considerations, in first experiment (Set 1) aliquots were withdrawn from the reaction mixture (composed as described above) in various time intervals, immediately extracted with double volume of dichloromethane (DCM) and cooled to 25 °C. Fluorescence spectra of the DCM extracts of aliquots withdrawn at various time intervals are presented in Fig. 1A. According to that, fluorescence of the T* species of 2, centered at 524 nm, increases gradually, indicating progressing enzymatic cleavage of 1. φfl of 2 in DCM is 10.0% (Table 1), which enables satisfactory sensitivity of this approach even at the initial stages of reaction. On the basis of dependence of the T* fluorescence intensity on the reaction time (Fig. 1A inset), one can conclude that under the conditions applied, the enzymatic cleavage is practically completed during 10 hours.
| c BSA (M) | λ fl (nm) | φ fl (%) | k (h−1) | τ 1/2 | |
|---|---|---|---|---|---|
| a c BSA – concentration of BSA; λfl – wavelength of the T* fluorescence maxima of 2; φfl – quantum yield of fluorescence, measured relative to quinine bisulfate in 0.05 M H2SO4; k – rate constant of enzymatic cleavage; τ1/2 – time of half-reaction. | |||||
| Set 1 | 0 | 524 | 10.0 | 0.219 ± 0.001 | 3 h 10 min |
| Set 2 | 5.0 × 10−5 | 534 | 10.7 | 0.224 ± 0.002 | 3 h 20 min |
On the other hand, concentration of water in the local environment of fluorophore can be decreased by its incorporation into macromolecules containing hydrophilic fragments. From this point of view, relatively high affinity of flavonols to serum albumins36,37 seems to be beneficial. According to previous investigations,36 interaction of flavonol with BSA (bovine serum albumin) is accompanied by a substantial increase in the fluorescence intensity of T*. For this reason, we conducted second experiment (Set 2) in the presence of 5 × 10−5 M BSA with all other conditions kept identical as for Set 1. The concentration used corresponds to minimal amount of BSA needed to completely bind the flavonol derivative.36 The value of φfl of 2 under the latter conditions is practically the same as in DCM environment (Set 1, Table 1) and the course of enzymatic cleavage can also be monitored in a few minutes after start of reaction (Fig. 1B). Similarly to Set 1, more than 90% of the amount of 1 was transformed to 2 in 10 hours (Fig. 1B inset). Besides T* fluorescence rising at 534 nm with progress of enzymatic cleavage, weak blue fluorescence in the range 380–460 nm can be monitored under the conditions of Set 2. The latter emission presumably originates from BSA and 1 at the beginning and N* form36 of 2 (Scheme 1) at the end of the enzymatic cleavage process.
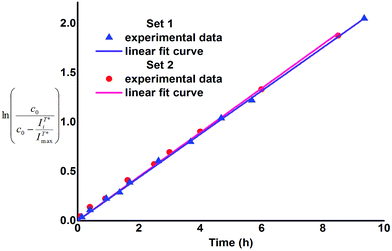
To find out if presence of BSA influences rate of the enzymatic cleavage of 1, kinetic parameters were evaluated and compared for both sets. Based on the assumption that intensity of the T* fluorescence corresponds to the concentration of 2, the first-order rate constants, characterizing both sets of the enzymatic reaction were calculated according to the equation:
 and
and  are the intensities of the T* fluorescence band maxima at ti and after 47 h, respectively; k is the rate constant of enzymatic cleavage. In the latter equation, ratio
are the intensities of the T* fluorescence band maxima at ti and after 47 h, respectively; k is the rate constant of enzymatic cleavage. In the latter equation, ratio  corresponds to concentration of 2 at time ti (in relative units) thus difference
corresponds to concentration of 2 at time ti (in relative units) thus difference  corresponds to concentration of 1 at time ti, in relative units. The values of k were assessed by plotting the left side expression of the above equation vs. time (Fig. 2).
corresponds to concentration of 1 at time ti, in relative units. The values of k were assessed by plotting the left side expression of the above equation vs. time (Fig. 2).
As one can notice, the above mentioned relationships demonstrate linear dependence (R2 > 0.99), which indicates that the enzymatic cleavage follows first-order kinetics and its rate constant does not change in the course of transformation of 1 to 2. Consequently, neither 1 nor 2 inhibit or activate β-glucosidase at the conditions applied and thus 1 can be considered as a benign fluorescent probe. Besides, the plots presented in Fig. 2 are very similar for both reaction sets and the obtained values of k and half-reaction times (τ1/2 = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/k) are practically the same within the range of experimental error (Table 1). Therefore, it can be concluded that presence of BSA (at concentration 5 × 10−5 M) does not influence the rate of enzymatic cleavage of 1. In our opinion, this finding can prospectively be of applicative interest as according to our research the use of BSA instead of organic solvent as fluorescence enhancing agent for the pair flavonol β-glucoside 1 – flavonol 2 allows to avoid extraction at measurement of enzymatic activity of β-glucosidase. Moreover, application of the Set 2 conditions allows to simplify sample preparation of the glucosidase enzymes probes for medical purposes. For instance, one of the stages of isolation and purification of the human acid β-glucosidase is separation from human serum albumin (HSA), conducted on the modified affinity columns.11 The latter stage thus seems to be unnecessary, if conditions of the Set 2 are applied. We intend to verify this assumption in our further work as well as apply other flavonol derivatives for the investigations of enzymatic cleavage, aimed at development of even more sensitive and rapid test-techniques.
2/k) are practically the same within the range of experimental error (Table 1). Therefore, it can be concluded that presence of BSA (at concentration 5 × 10−5 M) does not influence the rate of enzymatic cleavage of 1. In our opinion, this finding can prospectively be of applicative interest as according to our research the use of BSA instead of organic solvent as fluorescence enhancing agent for the pair flavonol β-glucoside 1 – flavonol 2 allows to avoid extraction at measurement of enzymatic activity of β-glucosidase. Moreover, application of the Set 2 conditions allows to simplify sample preparation of the glucosidase enzymes probes for medical purposes. For instance, one of the stages of isolation and purification of the human acid β-glucosidase is separation from human serum albumin (HSA), conducted on the modified affinity columns.11 The latter stage thus seems to be unnecessary, if conditions of the Set 2 are applied. We intend to verify this assumption in our further work as well as apply other flavonol derivatives for the investigations of enzymatic cleavage, aimed at development of even more sensitive and rapid test-techniques.
Conclusions
In this work we presented an optimized and simple approach for synthesis of a fluorescence probe from the group of flavonols, which is sensitive to the presence of β-glucosidase enzymes. Due to the ESIPT phenomenon, progress of the enzymatic cleavage of the probe can be monitored by the increasing red-shifted fluorescence near 530 nm. Enzymatic cleavage of the probe can be conveniently monitored at physiological conditions, if serum albumin (BSA) is used as specific fluorescence enhancer.Acknowledgements
This research was supported by the Ministry of Science and Higher Education grant DS/530-8457-D603-16. Financial support for the modernisation of chromatographic system utilized for sample analysis is acknowledged from the National Science Centre grant No. UMO 2012/05/B/ST5/01680.Notes and references
- H. Lodish, A. Berk, P. Matsudaira, C. A. Kaiser, M. Krieger, M. P. Scott, L. Zipursky and J. Darnell, in Molecular Cell Biology, W. H. Freeman, New York, 5th edn, 2003, pp. 73–79 Search PubMed.
- M. D. Cappellini and G. Fiorelli, Lancet, 2008, 371, 64–74 CrossRef CAS.
- K. S. Hruska, M. E. LaMarca, C. R. Scott and E. Sidransky, Hum. Mutat., 2008, 29, 567–583 CrossRef CAS PubMed.
- D. J. Mahuran, Biochim. Biophys. Acta, 1999, 1455, 105–138 CrossRef CAS.
- W.-Y. Jeng, N.-C. Wang, M.-H. Lin, C.-T. Lin, Y.-Ch. Liaw, W.-J. Chang, C.-I. Liu, P.-H. Liang and A. H.-J. Wang, J. Struct. Biol., 2011, 173, 46–56 CrossRef CAS PubMed.
- A. D. Junior, D. G. Borges, P. W. Tardioli and C. S. Farinas, Biotechnol. Res. Int., 2014, 2014, 1–8 CrossRef PubMed.
- A. Watanabe, M. Suzuki, S. Ujiie and K. Gomi, J. Biosci. Bioeng., 2016, 121, 259–264 CrossRef CAS PubMed.
- S. He and S. G. Withers, J. Biol. Chem., 1997, 272, 24864–24867 CrossRef CAS PubMed.
- Ch. Zhang, H. Yu, Y. Bao, L. An and F. Jin, Chem. Pharm. Bull., 2001, 49, 795–798 CrossRef CAS PubMed.
- S. Seshadri, T. Akiyama, R. Opassiri, B. Kuaprasert and J. K. Cairns, Plant Physiol., 2009, 151, 47–58 CrossRef CAS PubMed.
- G. A. Grabowski, S. Gaft, M. Horowitz and E. H. Kolodny, Crit. Rev. Biochem. Mol. Biol., 1990, 25, 385–414 CrossRef CAS PubMed.
- M. D. Graaf, I. C. v. Veen, I. H. v. d. Meulen-Muileman, W. R. Gerritsen, H. M. Pinedo and H. J. Haisma, Biochem. J., 2001, 356, 907–910 CrossRef PubMed.
- A. Zimran, T. Gelbart, B. Westwood, G. A. Grabowski and E. Beutler, Am. J. Hum. Genet., 1991, 49, 855–859 CAS.
- L. B. Daniels and R. H. Glew, Clin. Chem., 1982, 28, 569–577 CAS.
- E. Ponce, J. Moskovitz and G. Grabowski, Blood, 1997, 90, 43–48 CAS.
- T. Miura, L. Yuan, B. Sun, H. Fujii, M. Yoshida, M. Wakame and K. Kosuna, Biosci., Biotechnol., Biochem., 2002, 66, 2626–2631 CrossRef CAS PubMed.
- M. Rybus-Zając and I. Morkunas, Acta Agrobot., 2005, 58, 103–110 CrossRef.
- R. J. Chróst and J. Overbeck, Arch. Hydrobiol. Beih. Ergebn. Limnol., 1990, 34, 93–98 Search PubMed.
- W. Liu, J. Hong, D. R. Bevan and Y.-H. P. Zhang, Biotechnol. Bioeng., 2009, 103, 1087–1094 CrossRef CAS PubMed.
- D. E. Stott, S. S. Andrews, M. A. Liebig, B. J. Wienhold and D. L. Karlen, Soil Sci. Soc. Am. J., 2009, 74, 107–119 CrossRef.
- P. W. Stege, G. A. Messina, G. Bianchi and R. A. Olsina, J. Fluoresc., 2010, 20, 517–523 CrossRef CAS PubMed.
- S. Yan and G. Wu, Protein Pept. Lett., 2011, 18, 1053–1057 CrossRef CAS PubMed.
- J. R. Lakowicz, in Principles of Fluorescence Spectroscopy, Spinger US, 3rd edn, 2006, ch. 10, p. 365 Search PubMed.
- R. S. Sista, T. Wang, N. Wu, C. Graham, A. Eckhardt, T. Winger, V. Srinivasan, D. Bali, D. S. Millington and V. K. Pamula, Clin. Chim. Acta, 2013, 424, 12–18 CrossRef CAS PubMed.
- M. L. Graber, D. C. DiLillo, B. L. Friedman and E. Pastoriza-Munoz, Anal. Biochem., 1986, 156, 202–212 CrossRef CAS PubMed.
- J. E. Kwon and S. Y. Park, Adv. Mater., 2011, 23, 3615–3642 CrossRef CAS PubMed.
- S. Protti and A. Mezzetti, Photochemistry, 2012, 40, 295–322 CAS.
- O. M. Zamotaiev, V. Y. Postupalenko, V. V. Shvadchak, V. G. Pivovarenko, A. S. Klymchenko and Y. Mély, Org. Biomol. Chem., 2014, 12, 7036–7044 CAS.
- L. Feng, Z.-M. Liu, J. Hou, X. Lv, J. Ning, G.-B. Ge, J.-N. Cui and L. Yang, Biosens. Bioelectron., 2015, 65, 9–15 CrossRef CAS PubMed.
- S. Kumar and A. K. Pandey, Sci. World J., 2013, 2013, 1–16 Search PubMed.
- Z. Chen, S. Zheng, L. Li and H. Jiang, Curr. Drug Metab., 2014, 15, 48–61 CrossRef CAS PubMed.
- A. Kurzwernhart, W. Kandioller, É. A. Enyedy, M. Novak, M. A. Jakupec, B. K. Keppler and C. G. Hartinger, Dalton Trans., 2013, 42, 6193–6202 RSC.
- T.-L. Shih, C.-E. Chou, W.-Y. Liao and C.-A. Hsiao, Tetrahedron, 2014, 70, 3657–3664 CrossRef CAS.
- V. Semeniuchenko, Y. Garazd, M. Garazd, T. Shokol, U. Groth and V. Khilya, Monatsh. Chem., 2009, 140, 1503–1512 CrossRef CAS.
- A. J. G. Strandjord and P. F. Barbara, J. Phys. Chem., 1985, 89, 2355–2361 CrossRef CAS.
- J. Guharay, B. Sengupta and P. K. Sengupta, Proteins: Struct., Funct., Genet., 2001, 43, 75–81 CrossRef CAS.
- M.-X. Xie, M. Long, Y. Liu, C. Qin and Y.-D. Wang, Biochim. Biophys. Acta, 2006, 1760, 1184–1191 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: For synthetic routines, results of analyses, 1H and 13C NMR spectra. See DOI: 10.1039/c6ra06062e |
| This journal is © The Royal Society of Chemistry 2016 |