Amorphous silica nanoparticle-induced perturbation of cholesterol homeostasis as a function of surface area highlights safe-by-design implementation: an integrated multi-OMICS analysis†
Abstract
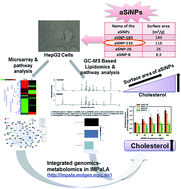
To close the knowledge gap between the wide application of amorphous silica nanoparticles (aSiNPs) and their health impact, the present study endeavored to investigate the molecular mechanisms involved in aSiNPs-mediated hepatotoxicity with a systems toxicology approach and how it is related to the physico–chemical properties of aSiNPs. To this end, we used four types of aSiNPs with different surface areas: aSiNP-116 (surface area: 116 m2 g−1), aSiNP-189 (surface area: 189 m2 g−1), aSiNP-26 (surface area: 26 m2 g−1) and aSiNP-8 (surface area: 8.3 m2 g−1); we also used the human hepatoma (HepG2) cell line as a model system. We applied multi-OMICS (DNA microarray based transcriptomics and GC-MS based lipidomics) followed by bioinformatics analysis in aSiNP-116 treated HepG2 cells. The perturbations of steroid-cholesterol biosynthesis were revealed by KEGG (with significantly altered genes) and IMPaLA (with integrated significantly altered genes and metabolites) pathway analysis. Furthermore, in corroboration with in silico analysis, the biochemical tests exhibited a concentration dependent increase in total cholesterol levels due to aSiNP-116 treatment. In a subsequent step, the hypothesis derived for aSiNP-116 was further tested for cells exposed to other aSiNPs (aSiNP-189, aSiNP-26 and aSiNP-8) with GC-MS based lipidomics as well as biochemical tests. The alterations in cholesterol biosynthesis were found to be directly proportional with the surface area of the aSiNPs, i.e., the larger the surface area, the higher the cholesterol level. Taken together, perturbation of cholesterol biosynthesis as a function of surface area was found to be a principal mode-of-action of aSiNPs exposure, which necessitates a safe-by-design approach for its biological applications.

 Please wait while we load your content...
Please wait while we load your content...