Synthesis and characterization of calcium lanthanum sulfide via a wet chemistry route followed by thermal decomposition
Yiyu Lia,
Lihua Zhangb and
Yiquan Wu*a
aKazuo Inamori School of Engineering, New York State College of Ceramics, Alfred University, 2 Pine Street, Alfred, New York 14802, USA. E-mail: wuy@alfred.edu; Tel: +1-607-871-2662
bCenter for Functional Nanomaterials, Brookhaven National Laboratory, Upton, New York 11973-5000, USA
First published on 31st March 2016
Abstract
Calcium lanthanum sulfide (CaLa2S4) has been extensively studied as a promising candidate for advanced infrared optical ceramics. In the present research, we report the successful synthesis of CaLa2S4 via a wet chemistry method followed by thermal decomposition. CaLa2S4 precursor material was first prepared by a facile ethanol-based wet chemical single-source precursor route. The precursor was then thermally decomposed in argon at high temperature to form CaLa2S4. The phase composition and morphology of the synthesized CaLa2S4 powder were confirmed and observed by X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM), respectively. Surface area and pore size analyses showed that the CaLa2S4 powder had a high specific surface due to a combined effect of small particle size and the existence of mesopores. Optical characterization revealed that the synthesized CaLa2S4 powder exhibited quantum size confinement and near-band-edge photoluminescence.
Introduction
Due to its favorable optical performance and mechanical properties, calcium lanthanum sulfide (CaLa2S4), crystallizing in the cubic Th3P4 crystal structure, has been extensively studied as a promising candidate for infrared optical ceramics.1,2 It has been reported that this alkaline earth and rare earth ternary sulfide compound, which displays a wide infrared transmittance range of 8–14 μm, has superior hardness and better rain erosion resistance compared with conventional infrared ceramic materials such as ZnS and ZnSe, indicating that it has the potential as an advanced infrared optical ceramic.3–5Since the 1980's, researchers have focused on developing various synthesis routes to prepare CaLa2S4 powders and ceramics. It has been reported that optical-quality CaLa2S4 powders can be synthesized via an alkoxide method followed by the sulfurization of precursors at high temperature in an atmosphere of H2S or CS2.6–9 Alternately, by adopting a carbonate precipitation route, M. Tsai et al. fabricated CaLa2S4 powders via CS2 sulfurization, and subsequently investigated the influence of varying the Ca/La stoichiometric ratio on the phase composition of the CaLa2S4 powders.10–13 W. White et al. developed an evaporative decomposition of solution (EDS) route for producing CaLa2S4, consisting of spraying an aqueous solution of Ca and La nitrates into a hot furnace and heating the resulting oxide powder in flowing H2S.14 In addition, researchers from the Office of Naval Research developed yet another method to fabricate CaLa2S4 powder, with high purity and large BET surface area, through flame spray pyrolysis of Ca(NO3)2 and La(NO3)3 followed by sulfurization in H2S.15 In summary, the previously investigated CaLa2S4 synthesis procedures have mainly employed different methods to prepare the calcium lanthanum precursors, followed by sulfurization at elevated temperatures in an atmosphere of CS2 or H2S. However, sulfurization is a very slow and time-consuming process, which requires the use of highly toxic and flammable gases such as CS2 and H2S, rendering it a costly and environmentally harmful route for synthesizing CaLa2S4.
The single-source precursor (one-pot) method has been extensively applied to synthesize various high-quality metal sulfide nanomaterials with controllable morphologies and sizes over the last two decades.16,17 Different sulfur-containing precursors, such as organometallic compounds, dithiocarbamate salts, trithiocarbonate salts, xanthate salts, and metal dialkyldithiophosphates have been employed to chelate metal ions with sulfur ions during wet chemical processes, with the corresponding sulfides subsequently obtained by pyrolysis of the precursors.18 Among them, it has been reported that sodium diethyldithiocarbamate and 1,10-phenanthroline have been successfully used as sulfur source and chelating agent for synthesizing various nano-sized semiconductor sulfides.19–23 However, few studies have reported the synthesis of CaLa2S4 through the use of a wet chemistry method followed by thermal decomposition.
In the present investigation, CaLa2S4 precursors were fabricated via a single-source precursor method, and CaLa2S4 powder was subsequently obtained through the thermal decomposition of the as-synthesized precursor material. The phase composition and morphology of the synthesized powder were determined and observed, respectively. The specific surface area and pore size distribution of the synthesized porous CaLa2S4 powder were measured and analyzed through Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively. Spectroscopic characterization was performed to develop an understanding of the correlation between the optical properties and the inherent physical and chemical properties of the synthesized CaLa2S4 powder.
Experimental
All employed chemicals were of analytical grade and were used as received without any purification. Fig. 1 shows a brief schematic of the process used to synthesize the CaLa2S4 powder. 20 mmol sodium diethyldithiocarbamate trihydrate (Na(Ddtc)·3H2O, Sigma-Aldrich) and 20 mmol 1,10-phenanthroline trihydrate ((Phen)·3H2O, ≥99%, Sigma-Aldrich) were first dissolved in separate 100 mL anhydrous ethanol (reagent alcohol, 100%, Decon), respectively. The alcoholic solutions of Na(Ddtc) and Phen were then mixed by vigorous stirring. In addition, 5 mmol lanthanum chloride heptahydrate (LaCl3·7H2O, 99%, Alfa Aesar) and 2.5 mmol calcium chloride dihydrate (CaCl2·2H2O, 99%, Alfa Aesar) were also dissolved in separate 100 mL anhydrous ethanol, respectively. Next, the lanthanum and calcium chlorides solutions were added dropwise into the mixed solution of Na(Ddtc) and Phen while being stirred, yielding a yellowish solution. 2 mL of thioglycolic acid (TGA, ≥98%, Sigma-Aldrich) was dissolved into 10 mL of anhydrous ethanol, and then added into the previously mixed yellowish alcoholic solution as a capping agent. After 1 hour of reaction, the resulting yellow precipitates were washed and centrifuged (Allegra X-12 Centrifuge, Beckman Coulter) several times with anhydrous ethanol and then dried in air at 55 °C for 24 hours. Finally, the dried precursor material was heat treated at 1000 °C for 5 hours in flowing argon to achieve thermal decomposition and reaction to form CaLa2S4.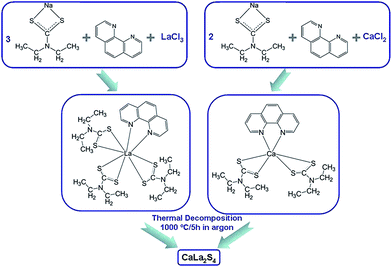 | ||
| Fig. 1 Schematic of the route used to synthesize CaLa2S4 via a wet chemistry method followed by thermal decomposition. | ||
The phase composition of the synthesized CaLa2S4 powder was determined by using XRD with Cu Kα (λ = 0.154 nm) radiation (Bruker D2 PHASER) at a voltage of 30 kV and a current of 10 mA. Measurement conditions of 0.03° 2θ step size and 0.2 s count time were employed over a measurement range of 10–75° 2θ. MDI Jade 9 was adopted for phase analysis, and Topas (Bruker) was used to perform Rietveld refinement of the XRD patterns. The morphological and microstructural features of the CaLa2S4 powder were investigated by SEM (FEI Quanta 200) with an acceleration voltage at 20 kV and TEM (JEOL 2100F) with an acceleration voltage at 200 kV. A Tristar II 3020 system (Micromeritics) was employed to measure the surface area and adsorption–desorption curve of the synthesized CaLa2S4 powder using BET and BJH methods, respectively. The powder was degassed at 25 °C for 10 min and at 150 °C for 1 hour prior to the measurement. Thermogravimetry (TG) and differential thermal analysis (DTA) (SDT Q600, TA Instruments) were conducted in a nitrogen (N2) atmosphere at a heating rate of 10 °C min−1 from 20 °C to 1100 °C with α-Al2O3 as reference. The band gap of the materials was determined using an ultraviolet-visible (UV-Vis) spectrophotometer (Perkin-Elmer Lambda 900) to measure the UV-Vis diffuse reflectance spectrum of the CaLa2S4 powder at 25 °C. Photoluminescence was investigated using photoluminescence spectra measurements (Jobin Yvon Fluorolog-3, Horiba) collected at 25 °C with a xenon lamp as the light source.
Results and discussion
Fig. 2 shows the XRD pattern of the CaLa2S4 precursor material after thermal decomposition and reaction at 1000 °C. The material is mostly single phase, with the main phase indexed to cubic calcium lanthanum sulfide (ICDD card no. 00-29-0339, I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3d, a = 8.6830 Å). Through Rietveld refinement, the lattice parameter of the material is refined to be 8.6814 Å, which is very close to the standard PDF card data. In addition, it should be noted that some minor peaks exist in the XRD pattern, which correspond to residual carbon remaining in the powder after thermal decomposition. The fact that the main phase of the powder is cubic CaLa2S4 demonstrates that the route applied in this research is a feasible and effective method to prepare CaLa2S4 without the use of any long sulfurization treatments using sulfur-containing gases. It also suggests that Na(Ddtc) and Phen are effective at chelating the La and Ca ions with sulfur ions during wet chemical precipitation. The effective chelation of the Ca, La, and S ions allows for the ternary sulfide CaLa2S4 to be obtained by subsequent thermal decomposition and reaction of the precursor material. Due to the relatively high temperature at which the thermal decomposition and reaction is performed, the material experiences particle growth, and as such the crystallite size of the reaction products is relatively high, apparent as the narrow peaks observed in the XRD pattern in Fig. 2.
3d, a = 8.6830 Å). Through Rietveld refinement, the lattice parameter of the material is refined to be 8.6814 Å, which is very close to the standard PDF card data. In addition, it should be noted that some minor peaks exist in the XRD pattern, which correspond to residual carbon remaining in the powder after thermal decomposition. The fact that the main phase of the powder is cubic CaLa2S4 demonstrates that the route applied in this research is a feasible and effective method to prepare CaLa2S4 without the use of any long sulfurization treatments using sulfur-containing gases. It also suggests that Na(Ddtc) and Phen are effective at chelating the La and Ca ions with sulfur ions during wet chemical precipitation. The effective chelation of the Ca, La, and S ions allows for the ternary sulfide CaLa2S4 to be obtained by subsequent thermal decomposition and reaction of the precursor material. Due to the relatively high temperature at which the thermal decomposition and reaction is performed, the material experiences particle growth, and as such the crystallite size of the reaction products is relatively high, apparent as the narrow peaks observed in the XRD pattern in Fig. 2.
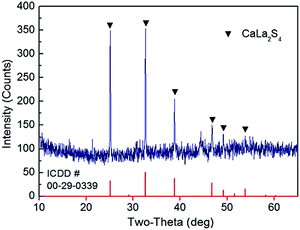 | ||
| Fig. 2 XRD pattern of the synthesized CaLa2S4 powder after thermal decomposition at 1000 °C for 5 hours in flowing argon. | ||
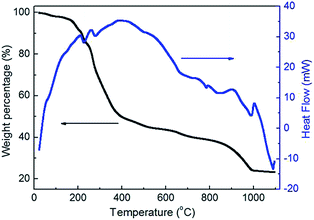
The TG/DTA curves of the CaLa2S4 precursor material obtained via the wet chemistry route are displayed in Fig. 3, which illustrates the various thermal reactions that the material goes through during the heat treatment in Ar. In the temperature range between room temperature and 400 °C, the broad exothermic peak accompanied by >50% weight loss corresponds to the rapid thermal decomposition of the polymers in the precursor. The two endothermic peaks in this range are likely due to the release of the waters of hydration from the hydrated nitrate raw materials used in the precursor. As temperature increases, the precursor material's rate of weight loss decreases. The weight loss of the CaLa2S4 precursor becomes approximately stable with a total weight loss of 79%, at the final temperature of 1000 °C, with an endothermic peak at 1000 °C corresponding to the formation of CaLa2S4. The TG/DTA curves shown here provide a basic reference for the thermal decomposition profile of the precursor to synthesize CaLa2S4.
The SEM image displayed in Fig. 4(a) shows that the CaLa2S4 powder obtained by thermal decomposition of the precursor consists of inhomogeneous submicron-sized agglomerates of particles. It is further suggested by TEM (Fig. 4(b)) that the CaLa2S4 particles are nanoscale, with an approximate average particle size of ∼50 nm. Detailed observations of the powder reveal neckings between adjacent particles, which can be attributed to the powder beginning to sinter at the relatively high heat treatment temperature. In addition, EDS measurements of the powder (shown in Fig. 4(c)) reveal the presence of the main cations and anions expected in CaLa2S4. The oxygen peak is due to the oxygen attached to the pores and surface of the CaLa2S4 powder. The presence of carbon indicates that some carbon species survive the thermal decomposition heat treatment of the CaLa2S4 precursor. The powder needs to be further purified to remove carbon impurities for infrared optical ceramic applications.
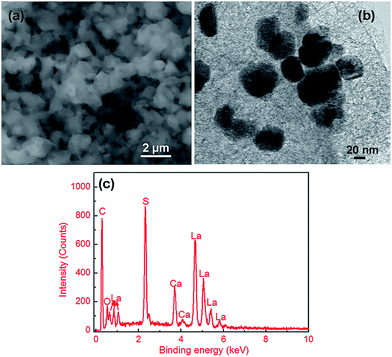 | ||
| Fig. 4 (a) SEM image, (b) TEM image, and (c) EDS spectrum of the CaLa2S4 powder synthesized via a wet chemistry method followed by thermal decomposition. | ||
Fig. 5(a) presents the N2 adsorption–desorption isotherm curve of the synthesized CaLa2S4 powder measured using the BJH method. The hysteresis loop shown in this typical irreversible type IV isotherm curve is due to pore condensation,24 suggesting the presence of mesopores within the CaLa2S4 powder, which are believed to form primarily during the thermal decomposition of the CaLa2S4 precursor. It is of interest to notice that no mesopores are shown in the particles in the TEM image. However, from the agglomerates observed in the SEM micrograph, the mesopores are speculated due to interspaces within the small nano-sized particles during the process of particles' stackings and agglomerations. The BET specific surface area of the sample was measured to be 75.82 ± 0.26 m2 g−1, which is attributed to the submicron-scale agglomerates of the nanoparticles, in combination with the effect of the mesopores. The pore size distribution curve displayed in Fig. 5(b) indicates that the sample has a narrow pore size distribution approximately at 23 nm, which also supports the notion that extensive mesopores exist within the sample.
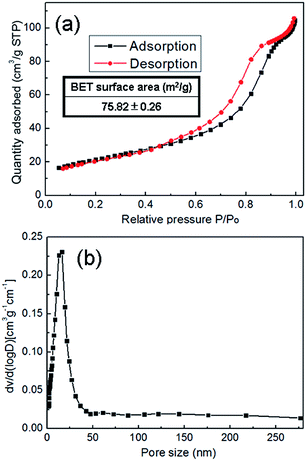 | ||
| Fig. 5 Adsorption–desorption isotherm curve (a) and pore size distribution curve (b) of the synthesized CaLa2S4 powder. | ||
The UV-Vis spectrum of the synthesized CaLa2S4 powder is shown in terms of absorbance versus wavelength in Fig. 6(a). The measured diffuse reflectance is converted to absorbance in this plot. The minimum wavelength required to promote an electron to the conduction band of the material is dependent on the material's band gap, and was estimated from the curve to be 429 nm. To determine the direct band gap of the CaLa2S4 powder, the curve can be replotted based on the equation for the Tauc relation shown below:25
| (αhν)m = A(hν − Egn) | (1) |
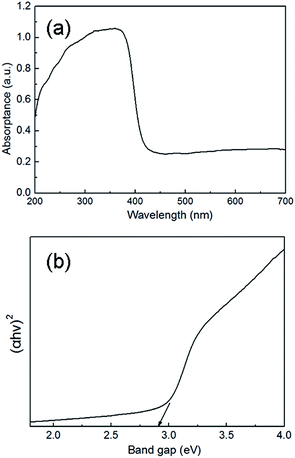 | ||
| Fig. 6 (a) UV-Vis absorption spectrum and (b) Tauc plot of the synthesized CaLa2S4 powder, with the UV-Vis spectrum collected at 25 °C. | ||
Fig. 7 shows the photoluminescence emission spectrum (excitation wavelength: 365 nm) of the synthesized CaLa2S4 powder measured with a xenon lamp as the light source. It was reported that the characteristic Mn2+ peak, which originated from Mn impurities in the CaS impurity phase present in CaLa2S4, was observed in the photoluminescence spectra of CaLa2S4 powder.27 However, there are no such impurity peaks in the spectra measured in this study, likely due to the difference in the employed processing routes. Here, the sample shows an emission peak at approximately 454 nm, which is assumed to be due to the near-band-edge photoluminescence of the CaLa2S4 semiconductor. It should be noted that the photoluminescence emission wavelength has a redshift compared with than the estimated wavelength required for electron promotion through light absorption, as determined through UV-Vis spectroscopy (shown in Fig. 6(a)), which suggests that the emitted photon energy is lower than the absorbed photon energy. The energy degradation here can be ascribed to the Stokes shift in energy (ΔStokes), which in semiconductors is attributed to the participation of phonons in the relaxation process.28 In addition, it is believed that some sulfur defects are formed during the thermal decomposition process which forms the CaLa2S4 powder. According to the semi-quantitative analysis based on EDS spectra measured in different areas of the powder, it is found that sulfur has an apparent deficiency compared with the value in the stoichiometry of CaLa2S4. Furthermore, it has also been reported that sulfur losses and deficiencies were detected in the material after hot pressing of CaLa2S4 ceramics in vacuum and argon.29,30 Thus, the broad peaks at 474 nm and 532 nm are assumed be due to the recombinations of electrons from conduction band and sulfur vacancy with holes in elemental sulfur species on surface, respectively.31
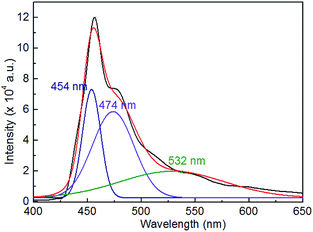 | ||
| Fig. 7 Photoluminescence emission spectrum of the synthesized CaLa2S4 powder measured under excitation at 365 nm at 25 °C. | ||
Conclusions
In the present study, the ternary sulfide CaLa2S4 was successfully synthesized by using a wet chemistry method (single-source precursor method) followed by thermal decomposition at 1000 °C for 5 hours in flowing argon. Analysis of the reaction products shows that the synthesized powder consists of submicron-scale agglomerates of nanoparticles, with cubic CaLa2S4 as the main phase. The CaLa2S4 powder possesses a large specific surface area due to the combination of small particle size and the presence of mesopores, which have a narrow size distribution approximately at 23 nm. It appears that the synthesized CaLa2S4 powder exhibits the quantum size effect, which in turn leads to band gap enhancement. Photoluminescence characterization indicates that the CaLa2S4 powder exhibits a near-band-edge photoluminescence emission with a Stokes shift and two broad emission bands due to the sulfur defects.Acknowledgements
We gratefully acknowledge the Office of Naval Research (ONR) (contract N00014-14-1-0546) for funding and supporting this research. This research used JEOL2100F TEM of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704.References
- J. Savage and K. Marsh, Proceedings of SPIE 0297: Emerging Optical Materials, International Society for Optics and Photonics, Bellingham, 1982 Search PubMed.
- K. Lewis, J. Savage, K. Marsh and A. Jones, Proceedings of SPIE 0400: New Optical Materials, International Society for Optics and Photonics, Bellingham, 1983 Search PubMed.
- J. Savage, K. Lewis and R. Whitehouse, Proceedings of SPIE 0588: Recent Developments in Materials & Detectors for the Infrared, International Society for Optics and Photonics, Bellingham, 1986 Search PubMed.
- K. Saunders, T. Wong, T. Hartnett, R. Tustison and R. Gentilman, Proceedings of SPIE 0683: Infrared and Optical Transmitting Materials, International Society for Optics and Photonics, Bellingham, 1986 Search PubMed.
- W. B. White, D. Chess, C. A. Chess and J. V. Biggers, Proceedings of SPIE 0297: Emerging Optical Materials, International Society for Optics and Photonics, Bellingham, 1982 Search PubMed.
- B. J. Tsay, L. H. Wang and M. H. Hon, Mater. Sci. Eng., B, 2000, 72, 31–35 CrossRef.
- Y. Han and M. Akinc, J. Am. Ceram. Soc., 1991, 74, 2815–2819 CrossRef CAS.
- L. H. Wang, M. H. Hon, W. L. Huang and W. Y. Lin, Mater. Sci. Eng., B, 1990, 7, 237–242 CrossRef.
- L. H. Wang and M. H. Hon, Jpn. J. Appl. Phys., Part 1, 1992, 31, 2177–2180 CrossRef CAS.
- L. H. Wang and M. H. Hon, Scr. Mater., 1992, 26, 455–459 CrossRef CAS.
- M. S. Tsai, L. H. Wang and M. H. Hon, J. Am. Ceram. Soc., 1995, 78, 1185–1190 CrossRef CAS.
- M. S. Tsai and M. H. Hon, Scr. Mater., 1995, 32, 713–718 CrossRef CAS.
- M. S. Tsai and M. H. Hon, J. Mater. Res., 1994, 9, 2939–2943 CrossRef CAS.
- D. L. Chess, C. A. Chess and W. B. White, J. Am. Ceram. Soc., 1983, 66, c205–c206 CrossRef CAS.
- S. S. Bayya, W. Kim, J. S. Sanghera, G. R. Villalobos and I. D. Aggarwal, US Pat., 20110174989 A1, 2010.
- T. Trindade, P. O'Brien, X.-m. Zhang and M. Motevalli, J. Mater. Chem., 1997, 7, 1011–1016 RSC.
- S. L. Castro, S. G. Bailey, R. P. Raffaelle, K. K. Banger and A. F. Hepp, J. Phys. Chem. B, 2004, 108, 12429–12435 CrossRef CAS.
- T. Duan, W. Lou, X. Wang and Q. Xue, Colloids Surf., A, 2007, 310, 86–93 CrossRef CAS.
- W. Han and M. Gao, Cryst. Growth Des., 2008, 8, 1023–1030 CAS.
- Y. Zhang, J. Lu, S. Shen, H. Xu and Q. Wang, Chem. Commun., 2011, 47, 5226–5228 RSC.
- S. Kozyukhin, N. Markova, A. Fairushin, N. Kuz'mina and E. Voronkov, Inorg. Mater., 2004, 40, 791–796 CrossRef CAS.
- C. J. Barrelet, Y. Wu, D. C. Bell and C. M. Lieber, J. Am. Chem. Soc., 2003, 125, 11498–11499 CrossRef CAS PubMed.
- P. Li, H. Li and W. Jie, J. Rare Earths, 2011, 29, 317–320 CrossRef CAS.
- S. Lowell, J. E. Shields, M. A. Thomas and M. Thommes, Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density, Kluwer Academic Publishers, Dordrecht, 2012 Search PubMed.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi B, 1966, 15, 627–637 CrossRef CAS.
- D. R. Rossington, R. A. Condrate and R. L. Snyder, Advances in Materials Characterization, Springer, New York, 2012 Search PubMed.
- J. Covino, D. C. Harris, M. E. Hills, R. T. Loda and R. W. Schwartz, Proceedings of SPIE 0505: Advances in Optical Materials, International Society for Optics and Photonics, Bellingham, 1984 Search PubMed.
- B. Ullrich, D. Ariza-Flores and M. Bhowmick, Thin Solid Films, 2014, 558, 24–26 CrossRef CAS.
- D. W. Roy, Proceedings of SPIE 0297: Emerging Optical Materials, International Society for Optics and Photonics, Bellingham, 1982 Search PubMed.
- J. Beswick, D. Pedder, J. Lewis and F. Ainger, Proceedings of SPIE 0400: New Optical Materials, International Society for Optics and Photonics, Bellingham, 1983 Search PubMed.
- C. Ye, X. Fang, M. Wang and L. Zhang, J. Appl. Phys., 2006, 99, 063504 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |