Naphthothiazole-based highly selective and sensitive fluorescent and colorimetric chemosensor for detection of pollutant metal ions†
Abstract
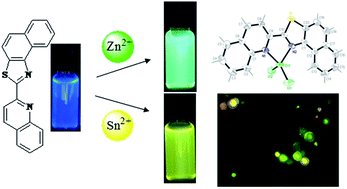
A new fluorescent and colorimetric chemosensor based on naphthothiazole S1 was synthesized and its applications as chemosensor for the selective sensing of pollutant metal ions in water resources and/or biological systems were investigated. Sensor S1 showed great selectivity for Zn2+ and Sn2+ over other metal ions. The Job's plot showed a 1 : 1 stoichiometry between S1 with Zn2+ and Sn2+. Interaction mode of sensor S1 with Zn2+ ion was further approved by single crystals X-ray crystallography method. The detection limit for the fluorescent chemosensor S1 toward Zn2+ and Sn2+ were respectively 2.60 × 10−8 M and 8.21 × 10−8 M.

 Please wait while we load your content...
Please wait while we load your content...