Synthesis of five metal based nanocomposite via ultrasonic high temperature spray pyrolysis with excellent antioxidant and antibacterial activity
Gopalu Karunakaran*ab,
Andrey Grigorjevich Yudina,
Matheswaran Jagathambalc,
Arup Ratan Mandala,
Nguyen Van Minha,
Alexander Gusevad,
Evgeny Kolesnikova and
Denis Kuznetsova
aDepartment of Functional Nanosystems and High-Temperature Materials, National University of Science and Technology “MISiS”, Leninskiy Pr. 4, Moscow, 119049, Russia. E-mail: karunakarang5@gmail.com
bDepartment of Biotechnology, K. S. Rangasamy College of Arts and Science, Tiruchengode-637215, Tamil Nadu, India
cDepartment of Bio-chemistry/Bio-technology/Bio-informatics, Avinashilingam Institute for Home Science and Higher Education for Women, Mettupalayam Road, Bharathi Park Road, Coimbatore 641 043, India
dG. R. Derzhavin Tambov State University, 33, Internatsionalnaya Street, Tambov, 392000, Russia
First published on 5th April 2016
Abstract
A unique five metal (Zn, Cu, Ni, Fe, and Mg) based nanocomposite material was prepared via ultrasonication high temperature spray pyrolysis maintained at 1200 °C. The influence of different concentrations (0.001 M, 0.01 M, and 1 M) on size and crystalline phase were analyzed. The nanocomposite exhibited excellent antioxidant and antibacterial activity.
The exceptional properties of nanocomposites lead to great attention and wide opportunities in industry and research.1 Currently, nanocomposites are one of the most active research areas in materials science as they show novel and enhanced properties based on characteristics such as particle size, morphology, and distribution.2 Generally, nanocomposites are of three types: (i) ceramic, (ii) metal, and (iii) polymer. Ceramic-based nanocomposites primarily consist of SiO2 with Al2O3 or with Ni for the production of high energy materials and to improve toughness.3 Metal-based nanocomposites primarily consist of a metal or its alloy (such as Fe–Cr with Al2O3) which is mainly used to enhance a material's strength and hence, leads to various applications in the automobile industry and aerospace.4 The polymer-based nanocomposites are made of polymer or polyester incorporated with silicates, carbon nanotubes, etc. for applications such as flame retardants, biomedical, and electronics.5
In general, there are different methods for production of metal-based nanocomposites such as sol–gel,6 CVD (chemical vapor deposition),7 ultrasonication,8 rapid solidification,9 liquid infiltration,10 and spray pyrolysis.11 However, amongst these methods, spray pyrolysis is the best for large scale production of metal nanocomposites,11 as it doesn't require any reducing, stabilizing, or capping agents for its production. Metals such as Zn, Cu, Ni, Fe, and Mg have applications in various fields including photo reactive devises, electrical, capacitance, photo degrading, and biomedical.12–16 Thus, the unique distinctive properties of the metals led us to develop a nanocomposite with all five metals. Hence, in the present study, we primarily focused on the production of the five metal based nanocomposite using ultrasonication high temperature spray pyrolysis. In addition, each produced nanocomposite was analyzed for its antioxidant and antibacterial activity using standard protocols.
AR grade zinc nitrate (ZnNO3), copper nitrate (CuNO3), nickel nitrate (NiNO3), iron nitrate (FeNO3), and magnesium nitrate (MgNO3) were used for preparation of precursor solutions (Fig. 1(a)) of different concentrations such as 0.001 M, 0.01 M, and 1 M respectively, in water (Milli-Q Ultrapure Water Solutions, Type 1, Merck Millipore, Darmstadt, Germany). The green colored solution in Fig. 1(a) is the precursor solution used in the spray pyrolysis experiment. The complete experimental setup and schematic representation of the ultrasonication high temperature spray pyrolysis is shown in Fig. 1 and 2. Each of the precursor solutions were filtered and the filtrate was then filled in the ultrasonic generator (DK 9 – 36 Mist Maker), which is shown in Fig. 1(b). The ultrasonic generator produces an aerosol which is allowed to flow into the silica reactor at an air flow rate of 16 L min−1. The diameter and length of the silica reactor is 25 mm and 500 mm, which is maintained by the two flask-based pump (KNF Berger D7911) as represented in Fig. 1(f) and (g). The reactor was maintained at a temperature of 1200 °C in a tube furnace (Nabetherme 20/250/13) which is shown in Fig. 1(c). The ultrasonic generator was allowed to continue until the solution finished. Once the solution in the ultrasonic generator was completely gone, the reactor was allowed to come to normal room temperature. Once room temperature was achieved, the powder was collected from the powder collection chamber (Fig. 1(e)). The powder collection chamber consists of an inlet, inner filter, chamber and outlet. During spray pyrolysis, the particles from the furnace are filtered by the inner filter and deposited in the powder collection chamber. Thus, after completion of the experiment the top of the powder collection chamber is opened and the powder is collected.
 | ||
| Fig. 2 Schematic diagram – showing the production of nanocomposites at different concentrations via ultrasonic high temperature spray pyrolysis experiment. | ||
The obtained powders were further characterized using several techniques. The nature and crystalline phase of the nanocomposite samples were identified by X-ray powder diffraction patterns (Difray, 401, Russia, Saint Petersburg). Elemental groups and chemical bonds in the nanocomposites were investigated using a Fourier transform infrared (FTIR) spectrophotometer (Thermo Scientific, Nicolet 380, USA). A scanning electron microscope (SEM – JEOL, JSM – 6610 LV, Japan) was used to determine the dimensions and form of the nanocomposites. The presence of elements was confirmed by energy dispersive spectrum EDS; (EDX SSD X-MAX, Japan) and inductively coupled plasma atomic emission spectroscopy (ICP-AES; iCAP 6300 Radial View Company, Thermo Fisher Scientific Inc., United States).
The antioxidant activity of the nanocomposites was analyzed using an available DPPH method.17 Antibacterial activity of the synthesized AgNPs was determined against selected Gram positive and Gram negative clinical bacterial strains (collected from NCIM, India) such as Staphylococcus aureus (NCIM 2127) and Escherichia coli (NCIM 2065) using the Kirby–Bauer disk diffusion method.18 The prepared nanocomposites and positive control streptomycin at 100 μg mL−1 suspension were loaded onto the disk under aseptic condition. The plates were subsequently incubated at 37 °C for 24 to 48 hours after which the zone of inhibition (in mm diameter) was measured and tabulated.
Fig. 3(i) shows the XRD patterns of the synthesized nanocomposite. Broad peaks at ∼38° (220), ∼55° (311), ∼66° (400), ∼90° (511), and ∼100° (440) confirms the cubic nature of the particles which is in good agreement with Mg0.25Cu0.25Zn0.5Fe2O4 phase JCPDS file no. 00-051-0384 for nanocomposites obtained at 1 M concentration.19 The crystalline size of the particles was found to be 14.5 nm for particles obtained at 1 M concentration. However, it is interesting to note that the intensity of peaks at (311), (400), and (440) increased greatly and became sharper on increasing the concentration from 0.001 M to 1 M due to the increase in crystalline size of the particles.
The FTIR spectrum illustrated in Fig. 3(ii) clearly shows the presence of different elements and corresponding functional groups. The IR spectrum of the nanocomposites has shown absorption bands at 1037, 1317, 1523, 1662, and 3546 cm−1. The strong band at 3546 cm−1 may result from the O–H stretching vibration and be related to water molecules. The prominent bands at 1037, 1317, 1523, and 1662 cm−1 can be assigned as absorption bands, stretching vibration, and bending vibration of different metals present in the nanocomposites. The SEM image (Fig. 4) represents the presence of particles with spherical morphology with an average particle size of 156 nm at 0.001 M, 365 nm at 0.01 M, and 916 nm at 1 M concentration. It is clear that the particle size of the nanocomposites increases with an increase in the concentration of the precursor solution. This is due to the movement of aerosol and the aggregate formation in the reactor. Analysis using EDS confirmed the presence of all the five metals (Fig. 5(i)–(iii)) where the vertical axis displays the X-ray counts and the horizontal axis displays the keV. In addition, by ICP-AES, the mass percentage of the elements present in the nanocomposites was analyzed and the results are shown in Table 1.
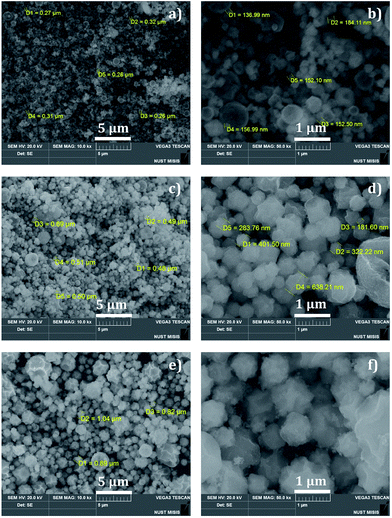 | ||
| Fig. 4 SEM image of nanocomposites under different magnifications (5 and 1 μm scale bar): (a and b) 0.001 M, (c and d) 0.01 M, and (e and f) 1 M. | ||
| Analyte | Spectral lines (nm) | Mass percentage (%) | ||
|---|---|---|---|---|
| 0.001 M | 0.01 M | 1 M | ||
| Zn | 202.5 | 14.4 | 16.5 | 17.7 |
| Cu | 224.7 | 14.0 | 15.3 | 16.3 |
| Ni | 221.6 | 13.0 | 14.8 | 16.0 |
| Fe | 259.9 | 13.1 | 14.0 | 15.5 |
| Mg | 279.5 | 3.54 | 4.20 | 4.61 |
The free radical scavenging activity of prepared nanocomposites is shown in Fig. 5(iv). It was observed that as the concentration of the nanocomposite is increased, its scavenging activity also increased respectively for 0.001 M nanocomposites: 20% (1 mg), 42% (5 mg), 66% (10 mg), and 72% (100 mg).
For 0.01 M nanocomposites: 24% (1 mg), 48% (5 mg), 72% (10 mg), and 78% (100 mg). However, for 1 M nanocomposites: 30% (1 mg), 53% (5 mg), 74% (10 mg), and 84% (100 mg). Similar antioxidant activity was reported for iron and nickel oxide nanoparticles.20,21 In living systems, free radicals are generated continuously due to various biochemical reactions. Nanocomposites will be very helpful to scavenge these free radicals.22 The antibacterial activity of the nanocomposites is shown in Fig. 6 and Table 2. It is clear that all the prepared nanocomposites have shown excellent antibacterial activity against both the bacteria due to the electrostatic interaction between the bacterial cell wall and nanocomposites.23 Hence, these nanocomposites can be used as an additive for various biomedical applications.
 | ||
| Fig. 6 Antibacterial activity of nanocomposites ((A): 0.001 M; (B): 0.01 M; (C): 1 M; (D): streptomycin). | ||
| Microorganisms | Zone of inhibition [mean ± SD (mm)] | |||
|---|---|---|---|---|
| Streptomycin | 0.001 M | 0.01 M | 1 M | |
| S. aureus | 13 ± 0.41 | 13 ± 0.60 | 14 ± 0.25 | 15 ± 0.32 |
| E. coli | 14 ± 0.12 | 13 ± 0.28 | 14 ± 0.36 | 15 ± 0.78 |
Conclusions
Metal (Zn, Cu, Ni, Fe, and Mg) based nanocomposite material was prepared using the corresponding nitrate precursor via ultrasonication high temperature spray pyrolysis maintained at 1200 °C. The obtained nanocomposite powder was characterized using several characterization techniques. Effect of concentrations such as 0.001 M, 0.01 M, and 1 M on the nanocomposite size and crystalline phase was analyzed. All three nanocomposites exhibited excellent antioxidant and antibacterial activities. Hence, the present investigation represents a novel approach to synthesize multiple nanocomposites via ultrasonic high temperature spray pyrolysis for biomedical applications.Acknowledgements
The authors gratefully acknowledge the financial support of the Ministry of Education and Science of the Russian Federation in the framework of Increase Competitiveness Program of NUST![[left double angle bracket]](https://www.rsc.org/images/entities/char_27ea.gif) MISiS
MISiS![[right double angle bracket]](https://www.rsc.org/images/entities/char_27eb.gif) (No. K4-2015-017).
(No. K4-2015-017).
Notes and references
- P. H. C. Camargo, K. G. Satyanarayana and F. Wypych, J. Mater. Res., 2009, 12, 1–39 CrossRef CAS.
- H. Shimada, T. Yamaguchi, T. Suzuki and H. F. Sumi, J. Power Sources, 2016, 302, 308–314 CrossRef CAS.
- A. Nakahira and K. Niihara, J. Ceram. Soc. Jpn., 1992, 100, 448–453 CrossRef CAS.
- S. Jia, D. Zhang, Y. Xuan and L. Nastac, Appl. Acoust., 2016, 103, 226–231 CrossRef.
- Y. Zare and I. Shabani, Mater. Sci. Eng., C, 2016, 60, 195–203 CrossRef CAS PubMed.
- J. Hua, Y. Liu, L. Wang, M. Feng, J. Zhao and H. Li, J. Magn. Magn. Mater., 2016, 402, 166–171 CrossRef CAS.
- S. S. Md Saleh and H. Md Akil, Fillers and Reinforcements for Advanced Nanocomposites, 2015, pp. 81–98 Search PubMed.
- P. Chitra, A. Muthusamy, R. Jayaprakash and E. R. Kumar, J. Magn. Magn. Mater., 2014, 366, 55–63 CrossRef CAS.
- S. S. Nayak, S. K. Pabi, D. H. Kim and B. S. Murty, Intermetallics, 2010, 18, 487–492 CrossRef CAS.
- A. Contreras, V. H. Lopez and E. Bedolla, Scr. Mater., 2004, 51, 249–253 CrossRef CAS.
- K. R. Nemade, R. V. Barde and S. A. Waghuley, Ceram. Int., 2015, 41, 4836–4840 CrossRef CAS.
- F. Al-Hazmi and F. Yakuphanoglu, J. Alloys Compd., 2015, 653, 561–569 CrossRef CAS.
- V. Demchenko, V. Shtompel and S. Riabov, Eur. Polym. J., 2016, 75, 310–316 CrossRef CAS.
- T. Menga, Q. Q. Xua, Y. T. Lia, X. Y. Xinga, C. S. Lib and T. Z. Rena, Electrochim. Acta, 2015, 155, 69–77 CrossRef.
- J. Yang, H. Chen, J. Gao, T. Yan, F. Zhou, S. Cui and W. Bi, Mater. Lett., 2016, 164, 183–189 CrossRef CAS.
- G. K. Meenashisundaram, M. H. Nai, A. Almajid and M. Gupta, Mater. Des., 2015, 65, 104–114 CrossRef CAS.
- A. Serpen, E. Capuano, V. Fogliano and V. Gokmen, J. Agric. Food Chem., 2007, 55, 7676–7681 CrossRef CAS PubMed.
- A. W. Bauer, W. M. Kirby, J. C. Sherris and M. Turck, Am. J. Clin. Pathol., 1966, 45, 493–496 CAS.
- B. E. Warren, X-Ray Diffraction, Addison-Wesley Publishing Company, Inc., Reading, Massachusetts, 1969 edition Search PubMed.
- S. Paul, J. P. Saikia, S. K. Samdarshi and B. K. Konwar, J. Magn. Magn. Mater., 2009, 321, 3621–3623 CrossRef CAS.
- J. P. Saikia, S. Paul, B. K. Konwar and S. K. Samdarshi, Colloids Surf., B, 2010, 78, 146–148 CrossRef CAS PubMed.
- G. Watal, A. Watal, P. K. Rai, D. K. Rai, G. Sharma and B. Sharma, Colloids Surf., B, 2013, 1028, 147–151 CAS.
- A. Abbaszadegan, Y. Ghahramani and A. Gholami, J. Nanomater., 2015, 720654 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |



