Photoinduced change in the shape of azobenzene-based molecular glass particles fixed in agar gel†
Ryota Ichikawa and
Hideyuki Nakano*
Department of Applied Chemistry, Muroran Institute of Technology, 27-1, Mizumoto-cho, Muroran, Hokkaido 050-8585, Japan. E-mail: nakano@mmm.muroran-it.ac.jp
First published on 31st March 2016
Abstract
Photomechanical behaviours of photochromic materials have been attracting a great deal of attention. Here we report a new photomechanical phenomenon, one in which azobenzene-based molecular glass particles fixed in an isotropic agar gel environment became elongated and formed string-like structures upon being irradiated with a linearly polarized laser beam. An analysis in three dimensions confirmed the direction of the elongation to be parallel to the polarization direction of the incident beam. The phenomenon could be explained by a photoinduced vibration and/or transport of the molecules parallel to the polarization direction of the incident beam to generate a force exerted by the particles to push the surrounding gel away in the direction parallel to the polarization direction. Photoinduced elongation was promoted by a high Tg for the material but impeded by the introduction of bulky substituents at both ends of the azobenzene moiety. Such elongation could also be induced by using linearly polarized incoherent LED light, suggesting that the coherence of the incident beam was irrelevant to the photomechanical behaviours.
Introduction
Photomechanical behaviours observed for photochromic materials have been attracting a great deal of attention. In early stages of the studies on a variety of photomechanical behaviours, photomechanical bending motions of azobenzene-based liquid-crystalline polymer films and fibres,1 reversible photomechanically induced changes in the shapes of needle- and plate-shaped microcrystals of photochromic compounds,2 and photo-induced surface relief grating (SRG) formation using azobenzene-based polymer films3 have been demonstrated. Photomechanically induced changes in the shape of colloidal spheres composed of azobenzene-conjugated polymers have recently been reported.4 Related photoinduced fluidization phenomena were also reported for azobenzene-based materials.5 Very recently, unique reversible changes in the shape of an agarose-based film containing photoreactive 1,4-bis(para-hydroxystyryl)benzene have been reported.6 We have been carrying out studies regarding the production of photochromic molecular glasses, namely amorphous glasses of photochromic materials based on low-molecular-mass derivatives,7–9 and have found them to display several kinds of photomechanical behaviours related to photoinduced mass transport. These behaviours include photoinduced SRG formation on their amorphous films,9,10 photomechanical bending motions of the molecular fibres with the bending direction dependent on the polarization direction of the incident beam,11 photoinduced mass flow at the surface of the amorphous films12 and photoinduced movements of glass fragments on the substrate.12,13 These phenomena could be explained by anisotropic mass transport induced in the direction parallel to the polarization direction of the incident laser beam. However, in these cases, the materials were placed in somewhat anisotropic environments that might result in anisotropic photomechanical behaviours. It is of interest and of importance to investigate the photomechanical behaviours in an isotropic environment, which would provide information about the exact direction of the photomechanical force and might help to elucidate the mechanism of the photomechanical behaviours related to the photoinduced mass transport. In the present study, we investigated photomechanical behaviours of the glass particles of azobenzene-based molecular materials, in particular 4-[bis(4-methylphenyl)amino]azobenzene (BMAB),7b,10 4-[phenyl-(biphenyl-4-yl)amino]azobenzene (PBAB),14 4-[di(biphenyl-4-yl)amino]azobenzene (DBAB),7b,10 4-[bis(9,9-dimethylfluoren-2-yl)amino]azobenzene (BFlAB)7b,9a,10 and 4,4′-bis[bis(4-methylphenyl)amino]azobenzene (BBMAB)7b (Fig. 1), fixed in an agar gel serving as an isotropic environment.Results and discussion
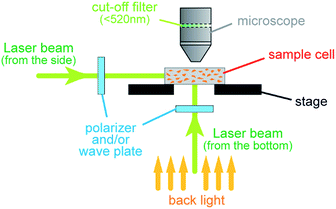
As we have already reported, the azobenzene-based materials BMAB, PBAB, DBAB, BFlAB and BBMAB readily form amorphous glasses with glass transition temperatures (Tg) of 27, 48, 68, 97 and 79 °C, respectively, and exhibit photochromism based on trans–cis and cis–trans isomerization reactions in their amorphous-film states.7b,14 The experimental set-up used for monitoring the photomechanical behaviours is illustrated in Fig. 2. Crushed powders of these materials were fixed in agar gel (concentration of agar: 2.5 mg cm−3) in a transparent sample cell, and the cell was placed on the stage of the optical microscope. Photomechanical behaviours of the particles with dimensions of ca. 5–15 μm were monitored at room temperature (ca. 21 °C) when these particles were irradiated with a polarized laser beam (488 nm) from either the bottom or side of the sample.Upon irradiation of the BFlAB particles in the agar gel with a linearly polarized laser beam, a new photomechanical behaviour was found. As shown in Fig. 3a, when the sample particles in the agar gel were irradiated from the bottom with the laser beam (15 mW),‡ the shapes of the particles were gradually and drastically changed, being elongated parallel to the polarization direction of the incident laser beam to form a string-like structure. The resulting structure was stable at room temperature after the irradiation ceased. When the resulting string-like particles were irradiated with the laser beam with another polarization direction, the particles immediately shrank and then elongated in the direction parallel to the new polarization direction. Such phenomena can be seen in ESI Videos 1 and 2.† It has been reported that the colloidal spheres with submicron dimensions composed of azobenzene-conjugated polymers placed on the surface of a silicon wafer were deformed upon irradiation with a linearly polarized laser beam to form ellipsoid structures.4 This phenomenon somewhat resembled the present photomechanical behaviours, but the previously obtained structures were quite different from the ones we obtained.
When the pristine sample was irradiated from the side, a similar elongation of the particles parallel to the polarization direction was observed without elongation in the direction parallel to the path of the laser beam (Fig. 3b). In this way, a three-dimensional analysis was used to confirm that the photomechanical elongation took place just in the direction parallel to the polarization direction of the incident laser beam in the isotropic environment. Similar photomechanical elongations were observed for BMAB, PBAB, DBAB and BBMAB particles fixed in agar gel. It is notable that such photoinduced elongation could not be observed for crystalline samples of Disperse Red 1 and 4-iodoazobenzene, which cannot form a glass, suggesting that a glass-forming ability at around room temperature is needed for the material to exhibit the photoinduced elongation behaviour.
The rates of elongation were also evaluated, by monitoring the change in the relative length of the long axis of the particle parallel to the polarization direction of the incident laser beam compared to the original length during irradiation of the beam under different conditions. Fig. 4 shows the change in the relative length of BFlAB particles upon irradiation with laser beams of various intensities. Although the data have somewhat large distributions, the rates of such photoinduced elongation were found to depend on the intensity of the incident laser beam, increasing with an increasing intensity of the incident laser beam. It was also found that the rate decreased in a harder gel; that is, by increasing the concentration of agar from 2.5 to 12.5 mg cm−3, the relative length of the BFlAB particle long axis decreased from 4.4 ± 0.8 to 2.8 ± 0.3 after a 60 min irradiation with a 15 mW laser beam.
 | ||
| Fig. 4 The change in the relative length of the long axis of BFlAB particles during irradiation with laser beams of various intensities. | ||
Photoinduced SRG formation observed for the amorphous films of azobenzene-based polymers and the amorphous molecular glasses upon interference irradiation has been reported to strongly depend on the polarization directions of the writing beams, and the mass transport was suggested to be induced in the direction parallel to the polarization direction of the laser beam.3,10 Related phenomena observed for azobenzene-based molecular materials such as photomechanical bending motions of the molecular fibres, photoinduced mass flow at the surfaces of the amorphous films and photoinduced movements of the glass fragments on the substrate were also found to depend on the polarization direction of the incident beam and could be explained by the vibration and/or transport of the molecules parallel to the polarization direction of the incident beam.12 Although the cause of such polarization-dependent vibration and/or transport of the molecules has not yet been clearly explained, the present photomechanical elongation of the particles can be explained as follows. We suggest that when the particles were irradiated with a linearly polarized laser beam, photoinduced trans–cis and cis–trans isomerization reactions of the molecules took place, resulting in a softening of the particles. Simultaneously, the molecules in the particles vibrated and/or moved in the direction parallel to the polarization direction of the incident beam. As a result, the particles pushed the surrounding gel away in the direction parallel to the polarization direction, resulting in elongation of the particles. This pushing force became stronger with the increase in intensity of the incident laser beam, but a harder gel strongly suppressed the elongation.
The rates of photoinduced elongation were also found to depend on the materials used, as shown in Fig. 5. With regard to monosubstituted azobenzenes, the rate increased in the order BMAB < PBAB < DBAB < BFlAB under the same conditions. We suggest that the present photomechanical elongation of the particles was related to both the frequency of trans–cis and cis–trans isomerization cycles and the fluidity of the material upon irradiation. That is, the increasing frequency of trans–cis and cis–trans isomerization cycles facilitated the vibration and/or transport of the molecules while the increasing fluidity of the material prevented the elongation due to surface tension and hence caused the particles to shrink. Since the photochromic properties of BMAB, DBAB, PBAB and BFlAB as amorphous films are assumed to be more or less similar to one another,7a,10 the fluidity was suggested to have the dominant effect on the photomechanical properties. The fluidity of the materials at a certain temperature upon irradiation was assumed to relate to the Tg values of the materials as discussed in our previous paper.10 That is, the fluidity upon irradiation was thought to be reduced by increasing Tg of the material before photoirradiation. Thus, the rate of elongation increased with increasing Tg of the material (BMAB < PBAB < DBAB < BFlAB). Fig. 6 shows the change in relative length for PBAB particles at a variety of temperatures. The results show that the rate decreased with increasing temperature. A presumed increase in the fluidity with increasing temperature may have contributed to the photomechanical elongation being more favourable at lower temperature. With regard to BBMAB, its elongation rate was lower than that for BMAB despite the considerably higher Tg of BBMAB relative to that of BMAB. Since the photochromic reactions of BBMAB were supressed in the amorphous solid due to bulky substituents at both ends of the azobenzene moiety,7b just a small photomechanical force could be induced. We previously reported that photoinduced SRG formation using photochromic amorphous molecular glasses was promoted by an increase in the Tg of the material but impeded by the introduction of bulky substituents at both ends of the azobenzene moiety.10 Thus, the results in the present study were consistent with those regarding the photoinduced SRG formation of the azobenzene-based molecular glasses, depending upon the material.
 | ||
| Fig. 5 The change in the relative length of the long axis of the particles of various azobenzene-based molecular glasses during irradiation with a laser beam having an intensity of 15 mW. | ||
 | ||
| Fig. 6 The change in the relative length of the long axis of the PBAB particles during irradiation with the laser beam at various temperatures. Intensity of the laser beam: 10 mW. | ||
It is expected that a variety of structures can be fabricated by controlling the photoirradiation conditions. Fig. 7a and b show the changes in the shape of BFlAB particles in agar gel upon irradiation with a circularly polarized laser beam from the bottom and from the left side of the sample cell, respectively. The results showed that the particle extended radially, perpendicular to the path of the incident beam, to form somewhat disk-like structures. Since the circularly polarized beam was assumed to be an assembly of linearly polarized beams with all polarization directions perpendicular to the path of the beam, the particles were suggested to expand to form such disk-like structures.
It is of interest to note that the photoinduced elongation behaviour could be observed when a linearly polarized incoherent LED light was used as the light source. When BFlAB particles fixed in agar gel were irradiated with incoherent LED light (450 nm, FWHM: 20 nm, intensity: 35 mW)§ through a polarizer, a change in shape involving elongation in the direction parallel to the polarization direction of the incident light was also found to take place, as shown in Fig. 7c. The average relative length of the long axis after 60 min-irradiation was 2.1 ± 0.3, being comparable to that upon irradiation with the coherent laser beam with a similar intensity. Thus, the coherence of the incident beam appeared to have been irrelevant to the photomechanical behaviours. These results were of importance for deriving the mechanism of the photomechanical behaviours.
Experimental
Materials
All azobenzene-based materials, i.e., BMAB, PBAB, DBAB, BFlAB and BBMAB, were prepared by the methods described in our previous papers.7b,9a,14 Agar was purchased commercially (Kanto Chemical Co., Inc.) and used without further purification.Sample preparation
Agar was dissolved in deionized water (2.5 or 12.5 mg cm−3) by heating the mixture at ca. 90 °C, and the resulting solution was poured into a transparent glass cell with a path length of 2 mm or 10 mm. The particles obtained by grinding the powder of the azobenzene-based materials were dispersed into the solution in the cell, and then the cell was cooled gradually at ambient atmosphere, followed by storage in a refrigerator (ca. 3 °C) to obtain the sample of the particles fixed in the agar gel.Methods for monitoring the photoinduced elongation and apparatus
The sample cell was placed on the stage of an optical microscope (Optiphot X2, Nikon) and the photomechanical behaviours of the particles with dimensions of ca. 5–15 μm were monitored at room temperature (ca. 21 °C) upon irradiation with a polarized laser beam (488 nm, CYAN-488-100 CDRH, SpectraPhysics Inc.) from either the bottom or side of the sample through appropriate polarizers and/or wave plates, as shown in Fig. 2. In order to investigate the temperature dependence of photomechanical behaviours, the temperature of the sample cell was controlled by using a TH-600 PM hot stage (Linkam) fitted on the stage of the microscope. As an incoherent light source, UHP-LED-450 (Prizmatix) was used.Conclusions
As a new photomechanical phenomenon, elongation of azobenzene-based molecular glasses fixed in an agar gel was found to occur upon irradiation with a linearly polarized laser beam. A three-dimensional analysis confirmed that the elongation took place parallel to the polarization direction of the incident beam. The phenomenon was suggested to be due to an induced vibration and/or transport of the molecules parallel to the polarization direction of the incident beam, generating a force pushing the surrounding gel away in the direction of the photomechanical elongation. Photoinduced elongation was promoted by a high Tg for the material but impeded by the introduction of bulky substituents at both ends of the azobenzene moiety. The results could be explained by the increased fluidity of the material upon irradiation and the photochromic reactivity in the amorphous glass. It was found that elongation of the particles can also be induced by using incoherent LED light. The result suggested that the coherence of the incident beam was irrelevant to the photomechanical behaviours. The present study provided important information for elucidating the mechanism of the photomechanical behaviours related to photoinduced mass transport and should help in the near-future development of a variety of photomechanical behaviours for use in practical applications such as photocontrollable micromotors and actuators.Acknowledgements
This research was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Photosynergetics” (No. 26107006) from MEXT, Japan.Notes and references
- Y. Yu, M. Nakano and T. Ikeda, Nature, 2003, 425, 145 CrossRef CAS PubMed; M. Kondo, Y. Yu and T. Ikeda, Angew. Chem., Int. Ed., 2006, 45, 1378 CrossRef PubMed; H. J. Choi, K.-U. Jeong, L.-C. Chien and M.-H. Lee, J. Mater. Chem., 2009, 19, 7124 RSC; C. L. van Oosten, C. W. M. Bastiaansen and D. J. Broer, Nat. Mater., 2009, 8, 677 CrossRef PubMed.
- S. Kobatake, S. Takami, H. Muto, T. Ishikawa and M. Irie, Nature, 2007, 446, 778 CrossRef CAS PubMed; R. O. Al-Kaysi, A. M. Muller and C. J. Bardeen, J. Am. Chem. Soc., 2006, 128, 15938 CrossRef PubMed; H. Koshima, N. Ojima and H. Uchimoto, J. Am. Chem. Soc., 2009, 131, 6890 CrossRef PubMed.
- P. Rochon, E. Batalla and A. Natansohn, Appl. Phys. Lett., 1995, 66, 136 CrossRef CAS; D. Y. Kim, S. K. Tripathy, L. Li and J. Kumar, Appl. Phys. Lett., 1995, 66, 1166 CrossRef; P. S. Ramanujam, N. C. R. Holme and S. Hvilsted, Appl. Phys. Lett., 1996, 68, 1329 CrossRef; P. Lefin, C. Fiorini and J.-M. Nunzi, Pure Appl. Opt., 1998, 7, 71 CrossRef; N. K. Viswanathan, D. Y. Kim, S. Bian, J. Williams, W. Liu, L. Li, L. Samuelson, J. Kumar and S. K. Tripathy, J. Mater. Chem., 1999, 9, 1941 RSC; T. Ubukata, T. Seki and K. Ichimura, Adv. Mater., 2000, 12, 1675 CrossRef; A. Natansohn and P. Rochon, Chem. Rev., 2002, 102, 4139 CrossRef PubMed.
- Y. Li, Y. He, X. Tong and X. Wang, J. Am. Chem. Soc., 2005, 127, 2402 CrossRef CAS PubMed; Y. Li, Y. He, X. Tong and X. Wang, Langmuir, 2006, 22, 2288 CrossRef PubMed; J. Li, L. Chen, J. Xu, K. Wang, X. Wang, X. He, H. Dong, S. Lin and J. Zhu, Langmuir, 2015, 31, 13094 CrossRef PubMed.
- H. Nakano, S. Seki and H. Kageyama, Phys. Chem. Chem. Phys., 2010, 12, 7772 RSC; A. Bobrovsky, K. Mochalov, A. Chistyakov, V. Oleinikov and V. Shibaev, J. Photochem. Photobiol., A, 2014, 275, 30 CrossRef CAS; C. Rianna, M. Ventre, S. Cavalli, M. Radmacher and P. A. Netti, ACS Appl. Mater. Interfaces, 2015, 7, 21503 Search PubMed; N. S. Yadavalli, S. Loebner, T. Papke, E. Sava, N. Hurduc and S. Santer, Soft Matter, 2016, 12, 2593 RSC.
- L. Zhang and P. Naumov, Angew. Chem., Int. Ed., 2015, 54, 8642 CrossRef CAS PubMed.
- (a) Y. Shirota, K. Moriwaki, S. Yoshikawa, T. Ujike and H. Nakano, J. Mater. Chem., 1998, 8, 2579 RSCD. Nagahama, T. Ujike, K. Moriwaki, S. Yoshikawa, H. Nakano and Y. Shirota, J. Photopolym. Sci. Technol., 1999, 12, 277 CrossRef CAS; Y. Shirota, H. Utsumi, T. Ujike, S. Yoshikawa, K. Moriwaki, D. Nagahama and H. Nakano, Opt. Mater., 2003, 21, 249 CrossRef; (b) T. Tanino, S. Yoshikawa, T. Ujike, D. Nagahama, K. Moriwaki, T. Takahashi, Y. Kotani, H. Nakano and Y. Shirota, J. Mater. Chem., 2007, 17, 4953 Search PubMed.
- H. Utsumi, D. Nagahama, H. Nakano and Y. Shirota, J. Mater. Chem., 2000, 10, 2436 RSC; H. Utsumi, D. Nagahama, H. Nakano and Y. Shirota, J. Mater. Chem., 2002, 12, 2612 RSC; D. Nagahama, H. Nakano and Y. Shirota, J. Photopolym. Sci. Technol., 2008, 21, 755 CrossRef CAS.
- (a) H. Nakano, T. Takahashi, T. Kadota and Y. Shirota, Adv. Mater., 2002, 14, 1157 CrossRef CAS; (b) H. Nakano, T. Takahashi, T. Tanino and Y. Shirota, Dyes Pigm., 2009, 84, 102 CrossRef.
- H. Nakano, T. Tanino, T. Takahashi, H. Ando and Y. Shirota, J. Mater. Chem., 2008, 18, 242 RSC; H. Nakano, Chem. Lett., 2011, 40, 473 CrossRef CAS.
- H. Nakano, J. Mater. Chem., 2010, 20, 2071 RSC; H. Nakano, R. Ichikawa and R. Matsui, Micromachines, 2013, 4, 128 CrossRef CAS.
- H. Nakano and M. Suzuki, J. Mater. Chem., 2012, 22, 3702 RSC.
- M. Suzuki and H. Nakano, J. Photopolym. Sci. Technol., 2012, 25, 159 CrossRef CAS.
- H. Ando, T. Tanino, H. Nakano and Y. Shirota, Mater. Chem. Phys., 2009, 113, 376 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Videos 1 and 2 with their legends. See DOI: 10.1039/c6ra05792f |
| ‡ ca. 1.7 W cm−2. |
| § ca. 270 mW cm−2. |
| This journal is © The Royal Society of Chemistry 2016 |