Catalytic remediation of phenol contaminated wastewater using Cu–Zn hydroxide nitrate†
Assadawoot Srikhaowa,
S. Meejoo Smith*bc,
Kanchana Uraisinc,
Komkrit Suttiponparnitd,
Chanapa Kongmarkef and
Chitiphon Chuaichamc
aMaterials Science and Engineering Graduate Program, Faculty of Science, Mahidol University, Rama VI Rd, Rajathevi, Bangkok, 10400, Thailand
bCenter of Sustainable Energy and Green Materials, Faculty of Science, Mahidol University, Rama VI Rd, Rajathevi, Bangkok, 10400, Thailand. E-mail: siwaporn.smi@mahidol.ac.th; Fax: +66-2-3547151; Tel: +66-2-22015164
cDepartment of Chemistry, Faculty of Science, Mahidol University, Rama VI Rd, Rajathevi, Bangkok, 10400, Thailand
dEnvironmental Research and Management Department, PTT Research and Technology Institute, 71 M.2 Phahonyothin Rd, Sanubtub, Wangnoi, Ayutthaya 13170, Thailand
eSynchrotron Light Research Institute, 111 University Avenue, Muang, P. O. Box 93, Nakhon Ratchasima, 30000, Thailand
fDepartment of Materials Science, Faculty of Science, Kasetsart University, 50 Ngam Wong Wan Rd, Ladyaow, Chatuchak, Bangkok, 10900, Thailand
First published on 7th April 2016
Abstract
This work highlights an application of Cu–Zn hydroxide nitrate (denoted as 6Cu–Zn) as a highly effective and reusable catalyst for catalytic wet peroxide oxidation of phenol under mild conditions (35 °C). PXRD and XANES experiments were carried out to confirm a single phase of copper hydroxide nitrate in the 6Cu–Zn sample. The catalytic activity of 6Cu–Zn for degrading phenol was reported in terms of the percent phenol conversion, and chemical oxygen demand (COD) removal efficiency. Treatment of 100, 200, and 500 ppm aqueous phenol solutions with H2O2/6Cu–Zn resulted in complete phenol degradation within 10 min. The 6Cu–Zn catalyst can be reused for up to five consecutive runs while maintaining complete phenol conversion and COD removal efficiency (greater than 90%) after water washing. This work introduces a simple, mild, energy efficient and effective pretreatment method for highly toxic wastewater which could be applied prior to feeding the pretreated wastewater to subsequent conventional treatment units.
1. Introduction
Phenol and its derivatives are employed in various industries including chemical, polymer, coal processing, pharmaceutical manufacturing and petrochemical processing.1 Conventionally, separation processes such as solvent extractions are employed to reduce the concentration of phenol in water, followed by effective removal of remaining phenol using solid sorbents.2 Although the solvents used in extraction processes are recyclable, additional treatment and disposal of spent solid sorbents is required. Bioremediations of phenol contaminated water are generally successful at low phenol concentrations (<50 ppm).3 When the phenol concentration in water exceeds 70 ppm, the contaminated water becomes highly toxic to microorganisms, inhibiting biological treatment processes.4 Integrated H2O2/iron salt treatments have shown great promise as effective chemical pretreatment methods for highly toxic wastewater.5 However, due to the high solubility of iron salts in water recovery of the metal salt catalysts from sludge waste is difficult, involving the use of heat and caustic chemicals6 or complex, expensive equipment.7 Consequently, utilizing heterogeneous catalyst–H2O2 integrated systems (so called catalytic wet peroxide oxidation or CWPO) is more attractive as these can be easily recycled and reused. Development of catalytic water remediation technologies for operation at lower (ambient) temperature and at atmospheric pressure is of considerable importance as this would greatly reduce the cost and energy use of the process. However there are only a limited number of CWPO catalysts, being employed in a few hours timeframe treatments of contaminated water with phenol concentration exceed 70 ppm at temperature ≤ 40 °C under atmospheric pressure. Karthikeyan and coworker8 prepared Fe–Co–Al oxide for the oxidation of 1000 ppm phenol at 30 °C, and phenol conversion of 97% was achieved after 1 h treatment of the solution with an initial pH 3.5. Furthermore, Zhang and coworker9 utilized iron based ferromagnetic nanoparticle as CWPO catalyst in degradation of 282 ppm phenol solution (initial pH = 3) at temperature 40 °C. Over 85% of phenol was effectively removed and the catalyst can be regenerated and recycled. However, other water quality indices, e.g. chemical oxygen demand (COD) or total organic carbon (TOC) values, was not discussed in the above reports. In addition, Xia and coworker10 synthesize magnetically separable γ-Fe2O3 silica nanocomposite via a nano-assembling method followed by calcination, and the magnetic material was reported as an effective CWPO catalyst for degradation of 200 ppm phenol solution (initial pH = 4) at 40 °C, giving maximum of total organic carbon removal efficiency of 78% after 120 min treatment. Also, Castro and coworker11 reported that 4 h treatment of 1000 ppm phenol solution (initial pH = 6) using copper complex loaded polybenzimidazole was required to obtain the best efficiencies in terms of phenol conversion of 72% and COD removal of 54%. According to these data, it would be beneficial to develop a CWPO catalyst system to remediate phenolic contaminated water at concentration exceed 70 ppm, which give high COD and TOC removal efficiencies in a wide range of pH at temperatures below 40 °C within a short time.It was recently reported that Zn loaded copper hydroxide nitrate can be synthesized via a hydrothermal method, and subsequently employed as an effective and reusable catalyst for the rapid degradation of methyl orange dye under ambient conditions without the requirement for additional oxidant or irradiation.12 Hence, it is of fundamental interest to examine the feasibility of utilizing Zn loaded copper hydroxide nitrate for the remediation of phenol contaminated water. This work examines the composition of the Zn loaded copper hydroxide nitrate, and explores the catalytic activity of 6Cu–Zn in degradation of phenol under various reaction conditions, however with the temperature and pressure maintained at 35 °C and at atmospheric pressure. Aqueous phenol solutions (concentration ≥ 100 ppm) were employed as simulated wastewater samples to examine the practicality of the application of CWPO treatment using Zn loaded copper hydroxide nitrate to remediate highly polluted industrial wastewater.
2. Experimental
2.1 Materials
All chemicals were of commercial grade and were used without further purification. Copper nitrate trihydrate (Cu(NO3)2·3H2O; AR grade; Univar), zinc oxide powder (ZnO; AR grade; Merck) were starting reagents used to prepare catalyst samples. Hydrogen peroxide (35% w/v H2O2; reagent grade; Qrëc), phenol (C6H5OH; AR grade; Merck), and manganese dioxide (MnO2; AR grade; Aldrich) were used in catalytic studies. De-ionized water was employed as the solvent throughout the experiments.2.2 Preparation and characterization of catalysts
Firstly, Cu–Zn hydroxide nitrate was synthesized through employing a previously reported metal oxide assisted route12 at 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 6
1 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Cu
1 Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn molar ratio, and the light blue solid products obtained are hereafter denoted as 4Cu–Zn and 6Cu–Zn, respectively. Structural characterization of both samples was then carried out using powder X-ray diffraction (PXRD, Bruker, AXS model D8 advance, Cu Kα radiation) to elucidate crystalline phases. X-ray absorption spectroscopy (XAS, Beamline 5.2 of the Synchrotron Light Research Institute, Thailand) was employed to identify compositions of Cu- and Zn-containing phases in the samples. The oxidation states and local structures of Cu and Zn species in the 6Cu–Zn sample were confirmed through Cu and Zn K-edge X-ray Absorption Near Edge Structure (XANES) spectra, measured in fluorescence mode, using a Ge (220) double-crystal monochromator. XANES data were analyzed by means of the Athena graphical interface13 based on the IFEFFIT program suite.14 Elemental analysis was conducted using inductively coupled plasma optical emission spectrometry (ICP-OES system Spectro CirosCCD, SPECTRO Analytical Instruments).
Zn molar ratio, and the light blue solid products obtained are hereafter denoted as 4Cu–Zn and 6Cu–Zn, respectively. Structural characterization of both samples was then carried out using powder X-ray diffraction (PXRD, Bruker, AXS model D8 advance, Cu Kα radiation) to elucidate crystalline phases. X-ray absorption spectroscopy (XAS, Beamline 5.2 of the Synchrotron Light Research Institute, Thailand) was employed to identify compositions of Cu- and Zn-containing phases in the samples. The oxidation states and local structures of Cu and Zn species in the 6Cu–Zn sample were confirmed through Cu and Zn K-edge X-ray Absorption Near Edge Structure (XANES) spectra, measured in fluorescence mode, using a Ge (220) double-crystal monochromator. XANES data were analyzed by means of the Athena graphical interface13 based on the IFEFFIT program suite.14 Elemental analysis was conducted using inductively coupled plasma optical emission spectrometry (ICP-OES system Spectro CirosCCD, SPECTRO Analytical Instruments).
2.3 Phenol remediation experiments
Aqueous phenol solutions (100–500 ppm) were employed as simulated phenolic wastewater models, and each condition of remediation studies were performed in triplicates. Oxidative treatments of 20 mL phenol solutions were performed with stirring in a 50 mL round bottom flask equipped with a reflux condenser and situated in a temperature controlled water bath. Initially, treatment of degassed phenol solution with 6Cu–Zn under vacuum was investigated to probe the adsorption capacity of phenol on the 6Cu–Zn solid sample. Next, a kinetic study of phenol removal after treatment at 35 °C, with H2O2 and 6Cu–Zn under ambient conditions, was undertaken. The effects of phenol concentration, catalyst loading, H2O2 dosage, pH and reaction temperature on the phenol removal efficiencies by the integrated peroxide treatments (H2O2/6Cu–Zn catalyst system), were investigated. For each of the H2O2/6Cu–Zn treatments the phenol solution temperature was controlled by using a water cooling system or heater to reach and maintain the desired temperatures (20, 35, or 50 °C). Once the desired temperature was obtained a certain quantity of H2O2 was mixed into the phenol solution, followed by dispersion of a weighed portion of solid catalyst into the mixture. High dosages of H2O2 were employed in this work to ensure the highly effective peroxide treatments of the phenol solutions and to reduce the treatment times. The oxidation reactions were terminated at specific intervals by addition of MnO2 powder to the reaction mixture to convert ˙OH into −OH, and to decompose remaining H2O2.15 After filtration of the solid, the filtrates were then collected and subjected to further analysis using high performance liquid chromatography (HPLC), chemical oxygen demand (COD) measurements, and ICP-OES.The concentrations of unreacted phenol were obtained through HPLC analysis (HPLC-HP 1100 series) a mobile phase of 40% acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mM acetate buffer (4
5 mM acetate buffer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with using a Hypersil GOLD C8 column. Aqueous standards having known concentrations of benzoquinone, hydroquinone and catechol (possible phenol degradation products) were subjected to HPLC analysis providing reference chromatograms. The phenol conversion, denoted as XPh, was calculated using eqn (1):
1) with using a Hypersil GOLD C8 column. Aqueous standards having known concentrations of benzoquinone, hydroquinone and catechol (possible phenol degradation products) were subjected to HPLC analysis providing reference chromatograms. The phenol conversion, denoted as XPh, was calculated using eqn (1):
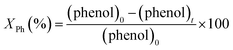 | (1) |
The COD of phenol solutions before, and after, treatment was measured using a standard closed reflux/colorimetric method.16 The COD removal efficiency is defined by the expression:
 | (2) |
2.4 Catalyst stability and reusability
The reusability and stability profile of the 6Cu–Zn catalyst was also investigated. The procedure employed in catalyst reuse experiments is given in the Scheme 1. A catalyst loading of 6 g L−1 was used in the first run to maintain an adequate amount of catalyst in subsequent cycles. After the first treatment, the suspension was centrifuged followed by decantation of the aqueous phase to obtain the spent catalyst. Next, the spent catalyst was washed with de-ionized water and dried at 100 °C in air for 1 h prior to reuse. Physical loss of catalyst through separation and washing (around 4–5 mg per treatment per run), was inevitable. ICP-OES was used to measure the degree of metal ion leaching from the catalyst to the solution, being indicative of catalyst stability. In addition, the spent solid catalyst after the 10th cycle was characterized by PXRD and XAS in order to study any changes in composition during extended cycling.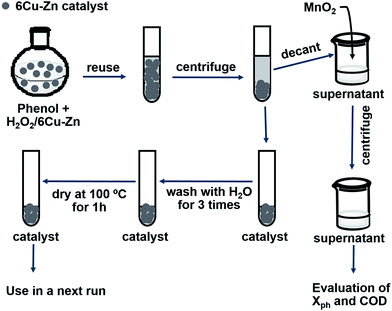 | ||
| Scheme 1 Experimental process to evaluate catalytic reusability and stability of 6Cu–Zn catalyst upon phenol oxidation. | ||
2.5 Reactivity and removal of leached metal ions
To evaluate whether the fast kinetics of phenol conversion is partly due to leached metal ions from solid 6Cu–Zn catalyst, additional experiments were performed to test the activity of solution after 2 hours of treatment. The resultant solution was filtered while hot (35 °C) to separate the solid catalyst and fresh phenol and hydrogen peroxide were added to obtain the initial concentrations required for repeating the reaction.Removal of metal ions leached in the solution after CWPO treatments was performed using hydroxide precipitation. Experiments were done in solutions after H2O2/6Cu–Zn of phenol for 2 hours, and solid catalysts were removed by filtration. Thereafter, 1.0 M NaOH (aq.) solution was added to the filtered solution resulting in precipitation of metal hydroxides. After removal of precipitates by filtration, the concentrations of Cu and Zn in the supernatant were determined on a Microwave plasma atomic emission spectrometer (Agilent technologies, 4200 MP-AES). The effect of pH in solution towards removal of Cu and Zn ions was investigated by varying the pH in the range from 5.5 (initial pH of phenol solution) to 11.
3. Results and discussion
3.1 Catalyst characteristics
Fig. 1(a)–(c) represent SEM images of the ZnO precursor, the 4Cu–Zn sample and the 6Cu–Zn sample, respectively. The ZnO powdered starting material, Fig. 1(a), contains primary particles having non-uniform sizes ranged between 0.1–1.5 μm in length. On the other hand, the 4Cu–Zn sample and 6 Cu–Zn have similar morphology, comprising of collections of merged plate-like grains, which are stacked on each other. The sizes of Cu–Zn hydroxide nitrate grains are ranged between 0.8–1 μm in length. The morphology of the Cu–Zn hydroxyl samples prepared in this work differs from the flower like catalyst derived from flower-liked ZnO particles reported in previous work,12 suggesting that the morphology of Cu–Zn hydroxide nitrates depends on the morphology of ZnO precursor. X-ray diffraction patterns of Cu–Zn hydroxide nitrates synthesized by different Zn loading contents, i.e. 4Cu–Zn and 6Cu–Zn, are illustrated in Fig. 2(a). No peak of crystalline impurities, i.e. hydroxides of Cu and Zn, was observed. All of the observed peaks matched well with the reported structure of Cu2(OH)3NO3 (the Joint Committee on Powder Diffraction Standards JCPDS file no. 74-1749). Nevertheless, it should be noted that diffraction peaks corresponding to Cu2O (111), CuO (11![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), including ZnO (100) and ZnO (101) appear in regions overlapping with the peaks observed in the prepared Cu–Zn hydroxide nitrate samples. Hence, X-ray absorption spectroscopy was employed to further probe the composition of 4Cu–Zn and 6Cu–Zn catalysts. Cu and Zn K-edges XANES spectra of catalysts and their corresponding reference compounds are presented in Fig. 2(b) and (c). It is worth noting that XANES spectrum of each compound exhibits specific features because the edge energy position, the white line peak position and the oscillation shapes of XANES spectrum are specifically sensitive to the oxidation states of elements and the local structure around the central absorbing atom (Cu and Zn atoms). Thus XANES spectra of the reference compounds can be used as a fingerprint to determine the unknown structure. From XANES spectra shown in Fig. 2(b) and (c), the edge energy positions of our hydroxide nitrate samples correlate well with those of Cu(II)O and Zn(II)O (ca. 8990 and 9663 eV, respectively) which would suggest that our samples mainly contain a mixture of Cu(II) and Zn(II) ions. In addition, no evidence of characteristic pre-edge features from either Cu2O (8980 eV) or CuO (8982 eV) was observed in Cu K-edges XANES of Cu–Zn hydroxide nitrates (Fig. 2(b)). It is therefore possible to conclude that Cu2O and CuO are not present in all synthesized samples. For Zn K-edge XANES in Fig. 2(c), the 6Cu–Zn sample exhibits distinctive post-edge spectral features (e.g. 9685, 9714, 9740 and 9760 eV), which would indicate that the local structure around Zn atoms in 6Cu–Zn should be different from that of ZnO. It is highly possible that Zn(II) ions might be incorporated into the Cu2(OH)3NO3 structure as it has been found in other hydroxyl double salt (HDS) structures, for example Ni1−xZn2x(OH)2(CH3CO2)2x·nH2O (0.15 < x < 0.25)17,18 and Pd(II) catalyst supported on the Ni–Zn mixed basic salt.19 This result also affirms the absence of a ZnO phase in the 6Cu–Zn sample, while a small amount of ZnO was found in the 4Cu–Zn sample as indicated by the XANES absorption arising from mixed phases of Cu–Zn hydroxide nitrate and ZnO. Additionally, this data suggests that the miscibility limit of Zn in Cu-hydroxide nitrate, prepared by the hydrothermal method, occurs at a Cu
), including ZnO (100) and ZnO (101) appear in regions overlapping with the peaks observed in the prepared Cu–Zn hydroxide nitrate samples. Hence, X-ray absorption spectroscopy was employed to further probe the composition of 4Cu–Zn and 6Cu–Zn catalysts. Cu and Zn K-edges XANES spectra of catalysts and their corresponding reference compounds are presented in Fig. 2(b) and (c). It is worth noting that XANES spectrum of each compound exhibits specific features because the edge energy position, the white line peak position and the oscillation shapes of XANES spectrum are specifically sensitive to the oxidation states of elements and the local structure around the central absorbing atom (Cu and Zn atoms). Thus XANES spectra of the reference compounds can be used as a fingerprint to determine the unknown structure. From XANES spectra shown in Fig. 2(b) and (c), the edge energy positions of our hydroxide nitrate samples correlate well with those of Cu(II)O and Zn(II)O (ca. 8990 and 9663 eV, respectively) which would suggest that our samples mainly contain a mixture of Cu(II) and Zn(II) ions. In addition, no evidence of characteristic pre-edge features from either Cu2O (8980 eV) or CuO (8982 eV) was observed in Cu K-edges XANES of Cu–Zn hydroxide nitrates (Fig. 2(b)). It is therefore possible to conclude that Cu2O and CuO are not present in all synthesized samples. For Zn K-edge XANES in Fig. 2(c), the 6Cu–Zn sample exhibits distinctive post-edge spectral features (e.g. 9685, 9714, 9740 and 9760 eV), which would indicate that the local structure around Zn atoms in 6Cu–Zn should be different from that of ZnO. It is highly possible that Zn(II) ions might be incorporated into the Cu2(OH)3NO3 structure as it has been found in other hydroxyl double salt (HDS) structures, for example Ni1−xZn2x(OH)2(CH3CO2)2x·nH2O (0.15 < x < 0.25)17,18 and Pd(II) catalyst supported on the Ni–Zn mixed basic salt.19 This result also affirms the absence of a ZnO phase in the 6Cu–Zn sample, while a small amount of ZnO was found in the 4Cu–Zn sample as indicated by the XANES absorption arising from mixed phases of Cu–Zn hydroxide nitrate and ZnO. Additionally, this data suggests that the miscibility limit of Zn in Cu-hydroxide nitrate, prepared by the hydrothermal method, occurs at a Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn mole ratio between 4
Zn mole ratio between 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 6
1 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Moreover, the incorporation of Zn atoms into the Cu hydroxide nitrate (Cu2(OH)3NO3) structure has been confirmed by ICP-OES analysis, 6Cu–Zn was found to contain 3.5% and 68% w/w of total Zn and Cu, respectively. The low Zn loading in 6Cu–Zn would suggest that the initial Cu
1. Moreover, the incorporation of Zn atoms into the Cu hydroxide nitrate (Cu2(OH)3NO3) structure has been confirmed by ICP-OES analysis, 6Cu–Zn was found to contain 3.5% and 68% w/w of total Zn and Cu, respectively. The low Zn loading in 6Cu–Zn would suggest that the initial Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn mole ratio of 6
Zn mole ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 produces a Cu–Zn hydroxide nitrate solid solution and a part of ZnO precursor converts to Zn(NO3)2 which is removed through washing and filtering. From the ratios studied, only 6Cu–Zn was further employed in phenol removal experiments.
1 produces a Cu–Zn hydroxide nitrate solid solution and a part of ZnO precursor converts to Zn(NO3)2 which is removed through washing and filtering. From the ratios studied, only 6Cu–Zn was further employed in phenol removal experiments.
 | ||
| Fig. 2 (a) Powder XRD patterns and XANES spectra of (b) Cu K-edge of 6Cu–Zn sample compared with Cu2O and CuO and (c) Zn K-edge of 6Cu–Zn sample, 4Cu–Zn and ZnO precursor. | ||
3.2 Phenol remediation
Fig. 3 shows that simple treatments of 100 ppm aqueous phenol solution with H2O2 or 6Cu–Zn, in separate experiments, under stirring with catalyst loading 3 g L−1 are ineffective as the obtained phenol removal efficiencies (or phenol conversions) and COD removal efficiencies were lower than 60% and 20%, respectively. From Fig. 3, it is noteworthy to point out the negligible phenol conversion and COD removal efficiency on 6Cu–Zn treatment under vacuum, compared with partial conversion and the low COD removal efficiency from similar treatment under ambient conditions. These results imply the low adsorption capacity of phenol over the 6Cu–Zn surface, as seen from the experiment conducted under vacuum. Additionally, oxidation of phenol can occur at the surface of 6Cu–Zn under ambient conditions without any requirement of additional oxidant. The oxidation under ambient atmosphere occurs through a catalytic wet oxidation (CWO) process, where the dissolved oxygen in the aqueous phenol solution acts as the oxidant. As seen in Fig. 3 together with HPLC results in ESI, Fig. S1(c),† a small amount of phenol was rapidly oxidized to hydroquinone after five minutes of the 6Cu–Zn treatment. It was suggested that using 6Cu–Zn alone is not sufficiently active to further oxidize hydroquinone under ambient conditions, as the conversion levels remain constant with time (10–120 min treatment) as shown in Fig. 3(a). Although relatively low conversions of phenol (100 ppm) were obtained when 6Cu–Zn acted as CWO catalyst, these are significantly higher than that of CWO catalyst system reported by Song et al.,20 that Co-doped Fe3O4 can catalyze phenol degradation under ambient pressure, however giving lower phenol conversion at higher temperature (20% after 30 min; 80 °C) compared with this study using Cu–Zn hydroxide nitrate (24% after 5 min, 35 °C). Additionally from Fig. 3(a), under ambient conditions, the initial rate of phenol removal by H2O2 treatment was found to be slightly faster than for 6Cu–Zn treatment (under ambient conditions), although the rate decreased after half an hour, as seen in Fig. 3(a). In the case of peroxide treatment, phenol is oxidized by generated hydroxyl radicals (˙OH). However, H2O2 alone is ineffective as a phenol oxidant within a 120 min timeframe (Fig. 3(b)) giving XPh of 58% and COD removal efficiency of 20%.Ideally, complete phenol removal should be a fast process to be industrially viable. Hence, the experimental parameters in this work were designed to monitor fast processes of phenol remediation, i.e. within 120 min. Phenol conversion (XPh) and COD removal efficiencies obtained from H2O2/6Cu–Zn treatment of 100 ppm phenol solution for 120 min are given in Fig. 4(a) and (b). These data implied that the H2O2 dosage in the H2O2/6Cu–Zn treatments primarily influenced the phenol degradation. An optimal H2O2 dosage is required as too high H2O2 concentrations may result in inferior performance, with the larger number of unstable ˙OH radical species reacting with each other rather than substrate.21,22 In addition, excessive amounts of catalyst resulted in slightly decreased performance, most likely due to fast H2O2 decomposition resulting in excessive amounts of ˙OH, which on reaction with water lead to formation of less active ˙HO2.23 Therefore, a catalyst loading of 3 g L−1 and H2O2 dosage of 3 mmol were selected as optimum parameters, achieving complete phenol conversion and high COD removal efficiency (>80%) within 2 h, and these were further employed in the subsequent phenol remediation studies.
The phenol conversion and COD removal efficiency results in Fig. 4(c), obtained from the H2O2/6Cu–Zn treatment (CWPO), indicated complete phenol conversion after only five minutes (phenol conversion reached 100%), and that phenol degradation products were further oxidized as seen from the increased COD removal efficiencies over time. The highest COD removal efficiency (92%) obtained from treatment of 100 ppm phenol solution after 120 min indicates a high degree of organic carbon mineralization in the phenolic wastewater. For the same phenol concentration (100 ppm), the rate of phenol removal by H2O2/6Cu–Zn treatment (35 °C; XPh = 98% after 5 min) was found to be much faster than H2O2/Fe/γ-Al2O3 treatment (50 °C; XPh = 86% after 90 min).24 Additionally, the CWPO of a less concentrated phenol solution (47 ppm) using LaFeO3 at 40 °C required 30 min to achieve XPh = 88%.25 Furthermore, treatment of 250 and 500 ppm aqueous phenol solutions with H2O2/6Cu–Zn under similar conditions to that shown in Fig. 4(c) resulted in complete phenol conversion after 10 min (Fig. 4(d)). In CWPO processes, the initial reaction is a conversion of CuII → CuI assisted by adsorbed H2O2 on the catalyst surface to generate ˙OH. Thereafter phenol degradation results from highly active ˙OH, affording degradation products or mineralization to CO2 and H2O. The high degree of phenol oxidation catalyzed by Cu–Zn hydroxide nitrate could result from ZnII species enhancing the redox properties of active CuII species in the catalyst.26,27
Incomplete COD removal is a result of residual phenol degradation products such as oxalic acid (detected by HPLC, ESI: Fig. S2†), which are difficult to oxidize. From this work, hydroquinone (after 10 min, 28% COD removal efficiency), and oxalic acid (after 2 h, 92% COD removal efficiency) were detected as degradation products. It is likely that phenol oxidation proceeds via a partial hydroxylation reaction, converting phenol to hydroquinone. Thereafter, ring opening and decarboxylation processes lead to formation of maleic acid, which is further oxidized to oxalic acid as an intermediate to CO2. This oxidation pathway is consistent with that proposed previously28,29 based on reactions conducted under high temperature and pressure. It was also observed that higher reaction rates can be attained at higher temperatures. In contrast, it is important to emphasize that H2O2/6Cu–Zn treatments does not require high temperatures, as COD removal efficiencies greater than 90% can be obtained under atmospheric pressure at 35–50 °C (ESI, Fig. S3†). These results indicate the practicality of H2O2/6Cu–Zn for CWPO of phenol under mild conditions, in contrast to other CWPO catalysts30,31 operating at higher temperatures (>50 °C), or under applied O2 partial pressures lower than 8 atm.28,32
Fig. 5(a) indicates that the 6Cu–Zn material is an effective CWPO catalyst for phenol degradation (optimum pH 5.5), and its high catalytic activity is maintained over a wide pH range (3–9) with complete phenol conversion and high COD removal efficiency (>80%). As the results, it can be concluded that 6Cu–Zn catalyst has a relatively wide effective operating pH range in comparison to those for Fe–Co–Al oxide,8 iron based ferromagnetic nanoparticle,9 γ-Fe2O3 silica nanocomposite.10 The lowering in performance under highly basic conditions, shown in Fig. 5(a), is possibly due to the presence of OH−, a radical scavenging agent23,27 as NaOH was used for pH adjustment. On the other hand, the slightly lower performance of 6Cu–Zn at pH 3 is probably due to catalyst leaching being more favorable under acidic conditions, as detected by ICP-OES (Fig. 5(b)).
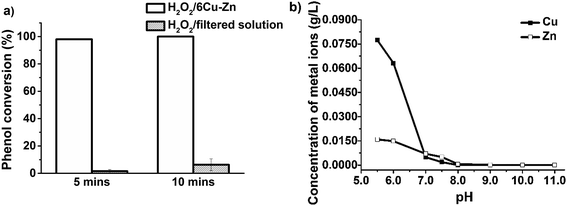
The catalyst leaching results may cast doubts on whether phenol degradation occurs via a heterogeneous process, or a homogeneous route catalyzed by dissolved metal ions in the phenol solution. Additional experiments were performed to examine whether the catalyst dissolution (leaching) played a major role regarding the fast kinetics of phenol oxidation during the H2O2/6Cu–Zn treatments, by using the filtered solution from the 1st cycle instead of 6Cu–Zn in the 2nd cycle, under the same reaction conditions reported in Fig. 4(c). The results in Fig. 6(a) indicate that the peroxide oxidation of phenol, in absence of the solid catalyst (filtered solution) resulted in phenol conversions of less than 10% after 10 min treatment, in comparison to 100% phenol conversion after 5 min heterogeneous treatment. Therefore, heterogeneous reactions are primarily responsible for CWPO of phenol in contrast to homogeneous processes.
The presence of acidic degradation products (i.e. oxalic acid detect by LC MS) may contribute to the enhanced solubility of metal species in treated phenolic solution, and the catalyst leaching could be suppressed by running the treatment under buffered reaction conditions. However, maintaining pH during water treatment may result in higher cost and be impractical, although pH adjustment before discharging to natural water reservoir, may be unnecessary as Cu–Zn hydroxide nitrate can effectively degrade phenol at pH 7. Treatment of buffer constituents (e.g. phosphate or acetate ions) may also need to be taken into account. In general, removal of heavy metals after pre-treatment of wastewater can be done by using simple ion exchange methods.33,34 Based on theoretical solubility of Cu- and Zn-hydroxide in aqueous solution,35 precipitation of both metal hydroxide species preferably occurs at neutral pH. To confirm that the metal ion due to Cu–Zn hydroxide nitrate leaching during CWPO pretreatment can be readily removed, a re-precipitation test has been performed. By slowly increasing the pH of the filtered solution (obtained from the 1st cycle of H2O2/6Cu–Zn treatment of 100 ppm phenol solution) from pH 5.5 to more basic, white fine particles started to form at pH 6 and the amount of fine particles obtained significantly increased at around neutral pH. Fig. 6(b) reports the monitored concentrations of Cu and Zn ions upon pH adjusting experiments, suggesting evidence for successful re-precipitation of metal ions at pH 7–8. These results add confidence that simple pH neutralization, a conventional step in water remediation, can eliminate the environmental concern of heavy metals due to Cu–Zn hydroxide nitrate leaching during CWPO pretreatment step. The results in Fig. 6(b) and S1 (ESI)† show that concentrations of Cu and Zn ions in supernatants decreased after hydroxide precipitation. The concentrations of both Cu and Zn ions in solutions after hydroxide precipitation at pH 8 meet the standard limit for Cu (1.3 ppm) and Zn (5 ppm) in drinking water by the U.S. EPA1 and the solution could be further supplied to a conventional process for wastewater treatment plants.
Catalyst systems for cost-effective wastewater remediation must show reusability and be stable under the process conditions. The results in Fig. 5(c) and 4(d) indicate that 6Cu–Zn can be reused for 6 cycles (2 h per cycle) after water washing (Scheme 1), providing a highly effective removal of organic compounds from contaminated water (COD removal efficiencies ∼ 90%). Albeit with lower activity, the spent catalyst could effectively remove phenol in later runs (7th to 9th). Further study of the stability of catalysts was also carried out. XRD and XANES data (Fig. 7) indicate the presence of a CuO phase in the spent catalyst after the 10th run, with a characteristic pre-edge XANES signal at 8982 eV and diffraction peaks at 32.5°, 35.5°, 38.8° and 48.8°. Therefore, the activity deterioration over time is likely due to the physical loss of catalyst upon washing and drying, and possibly due to the partial phase transformation (Cu2(OH)3NO3 → CuO).
 | ||
| Fig. 7 (a) XANES spectra of Cu K-edge of the 6Cu–Zn sample, spent 6Cu–Zn sample and CuO, and (b) PXRD patterns of the fresh 6Cu–Zn catalyst and the spent 6Cu–Zn sample after 10th run. | ||
4. Conclusion
A single phase Cu–Zn hydroxide nitrate can be successfully obtained through controlling the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn mole ratio during preparation of the material. The Cu–Zn hydroxide nitrate (6Cu–Zn) proved to be an effective CWPO catalyst for remediation of phenol contaminated water under ambient conditions, over a wide pH range, with a complete phenol conversion within 10 min, and with phenol degrading to oxalic acid after 2 h. According to a US EPA environmental assessment report,36 oxalic acid effectively undergoes fast (<1 day) aerobic and anaerobic biodegradation. Therefore, low-energy-consumption CWPO pretreatments over 6Cu–Zn may have the potential for integration into existing wastewater management systems, to lower the amount of toxic contaminants prior to further treatment employing conventional methods and subsequent discharge to the environment.
Zn mole ratio during preparation of the material. The Cu–Zn hydroxide nitrate (6Cu–Zn) proved to be an effective CWPO catalyst for remediation of phenol contaminated water under ambient conditions, over a wide pH range, with a complete phenol conversion within 10 min, and with phenol degrading to oxalic acid after 2 h. According to a US EPA environmental assessment report,36 oxalic acid effectively undergoes fast (<1 day) aerobic and anaerobic biodegradation. Therefore, low-energy-consumption CWPO pretreatments over 6Cu–Zn may have the potential for integration into existing wastewater management systems, to lower the amount of toxic contaminants prior to further treatment employing conventional methods and subsequent discharge to the environment.
Acknowledgements
This work was financially supported by the Thailand Research Fund through the Royal Golden Jubilee Ph. D Program (grant no. PHD/0119/2553) and the National Research Council of Thailand. Instrumentation was provided by the Synchrotron Light Research Institute, PTT Public Company Limited, and the Faculty of Science, Mahidol University. Authors also thank Prof. Chatvalee Kalambaheti, Prof. Atitaya Siripinyanond and Ms Weerawan Waiyawat for their technical assistance and valuable discussion.References
- A. Santos, P. Yustos, S. Rodriguez, E. Simon and A. Romero, Ind. Eng. Chem. Res., 2010, 49, 5583–5587 CrossRef CAS.
- S. Al-Asheh, F. Banat and L. Abu-Aitah, Sep. Purif. Technol., 2003, 33, 1–10 CrossRef CAS.
- B. K. Körbahti and A. Tanyolaç, Water Res., 2003, 37, 1505–1514 CrossRef.
- A. Garg, I. M. Mishra and S. Chand, Clean: Soil, Air, Water, 2010, 38, 27–34 CAS.
- A. K. De, B. K. Dutta and S. Bhattacharjee, Environ. Prog., 2006, 25, 64–71 CrossRef CAS.
- G.-m. Cao, M. Sheng, W.-f. Niu, Y.-l. Fei and D. Li, J. Hazard. Mater., 2009, 172, 1446–1449 CrossRef CAS PubMed.
- G. Zhang, Y. Gao, Y. Zhang and Y. Guo, Environ. Sci. Technol., 2010, 44, 6384–6389 CrossRef CAS PubMed.
- S. Karthikeyan, R. Boopathy, V. K. Gupta and G. Sekaran, J. Mol. Liq., 2013, 177, 402–408 CrossRef CAS.
- J. Zhang, J. Zhuang, L. Gao, Y. Zhang, N. Gu, J. Feng, D. Yang, J. Zhu and X. Yan, Chemosphere, 2008, 73, 1524–1528 CrossRef CAS PubMed.
- M. Xia, C. Chen, M. Long, C. Chen, W. Cai and B. Zhou, Microporous Mesoporous Mater., 2011, 145, 217–223 CrossRef CAS.
- I. U. Castro, D. C. Sherrington, A. Fortuny, A. Fabregat, F. Stüber, J. Font and C. Bengoa, Catal. Today, 2010, 157, 66–70 CrossRef CAS.
- A. Srikhaow and S. M. Smith, Appl. Catal., B, 2013, 130–131, 84–92 CrossRef CAS.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed.
- M. Newville, J. Synchrotron Radiat., 2001, 8, 322–324 CrossRef CAS PubMed.
- D. Zhao, B. L. Yi, H. M. Zhang and H. M. Yu, J. Membr. Sci., 2010, 346, 143–151 CrossRef CAS.
- APHA, AWWA' and WEF, Standard Methods for the Examination of Water and Wastewater, APHA, Washingtion, DC, 19th edn, 1998 Search PubMed.
- S. Yamanaka, K. Ando and M. Ohashi, MRS Proceedings, 1994, 371, 131 CrossRef.
- J.-H. Choy, Y.-M. Kwon, K.-S. Han, S.-W. Song and S. H. Chang, Mater. Lett., 1998, 34, 356–363 CrossRef CAS.
- T. Hara, M. Ishikawa, J. Sawada, N. Ichikuni and S. Shimazu, Green Chem., 2009, 11, 2034–2040 RSC.
- X. Song, Y. Zheng and H. Yin, J. Nanopart. Res., 2013, 15, 1–9 Search PubMed.
- R. Alnaizy and A. Akgerman, Adv. Environ. Res., 2000, 4, 233–244 CrossRef.
- R.-M. Liou, S.-H. Chen, M.-Y. Hung, C.-S. Hsu and J.-Y. Lai, Chemosphere, 2005, 59, 117–125 CrossRef CAS PubMed.
- S. K. Bhargava, J. Tardio, J. Prasad, K. Föger, D. B. Akolekar and S. C. Grocott, Ind. Eng. Chem. Res., 2006, 45, 1221–1258 CrossRef CAS.
- P. Bautista, A. F. Mohedano, J. A. Casas, J. A. Zazo and J. J. Rodriguez, J. Chem. Technol. Biotechnol., 2011, 86, 497–504 CrossRef CAS.
- J. Faye, E. Guélou, J. Barrault, J. M. Tatibouët and S. Valange, Top. Catal., 2009, 52, 1211–1219 CrossRef CAS.
- K. M. Valkaj, O. Wittine, K. Margeta, T. Granato, A. Katović and S. Zrnčević, Pol. J. Chem. Technol., 2011, 13, 28–36 Search PubMed.
- H. Ma, Q. Zhuo and B. Wang, Environ. Sci. Technol., 2007, 41, 7491–7496 CrossRef CAS PubMed.
- A. Quintanilla, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2010, 93, 339–345 CrossRef CAS.
- S. Zrnčević and Z. Gomzi, Ind. Eng. Chem. Res., 2005, 44, 6110–6114 CrossRef.
- N. Inchaurrondo, J. Cechini, J. Font and P. Haure, Appl. Catal., B, 2012, 111–112, 641–648 CrossRef CAS.
- C. Catrinescu, C. Teodosiu, M. Macoveanu, J. Miehe-Brendlé and R. Le Dred, Water Res., 2003, 37, 1154–1160 CrossRef CAS PubMed.
- F. Martínez, M. I. Pariente, J. Á. Botas, J. A. Melero and A. Rubalcaba, J. Chem. Technol. Biotechnol., 2012, 87, 880–886 CrossRef.
- D. Kołodyńska, Desalination, 2011, 267, 175–183 CrossRef.
- A. Dabrowski, Z. Hubicki, P. Podkościelny and E. Robens, Chemosphere, 2004, 56, 91–106 CrossRef CAS PubMed.
- K. F. Cherry, Plating Waste Treatment, Ann Arbor Sciences, Ann Arbor, MI, 1982 Search PubMed.
- The US Environmental Protection Agency (EPA), http://www.epa.gov/oppsrrd1/REDs/factsheets/4070fact.pdf.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra22326a |
| This journal is © The Royal Society of Chemistry 2016 |