ZnO–graphene–polyaniline nanoflowers: solution synthesis, formation mechanism and electrochemical activity†
Susanta Bera,
Atanu Naskar,
Moumita Pal and
Sunirmal Jana*
Sol-Gel Division CSIR-Central Glass and Ceramic Research Institute (CSIR-CGCRI) 196, Raja S. C. Mullick Road, P.O. Jadavpur University, Kolkata 700032, West Bengal, India. E-mail: sjana@cgcri.res.in; janasunirmal@hotmail.com; Fax: +91 33 24730957; Tel: +91 33 2473 3496
First published on 19th April 2016
Abstract
Three dimensional hierarchical inorganic–organic hybrid nanoflowers of conducting ZnO–chemically converted graphene–polyaniline nanocomposite have been successfully synthesized at low temperature from surfactant-free precursor solution and studied their formation mechanism. The nanocomposite with hierarchical architecture shows an enhanced electrochemical activity, illustrating a promise for application in electrochemical devices.
Hierarchical nanostructures (HNs) of inorganic–organic hybrid nanocomposites have recently drawn special attention for their extraordinary properties and wide spread applications in diverse areas such as energy storage and conversion, gas and bio-diagnostic sensing, catalysis and so on.1–5 However, the advancement in any functional property of a nanocomposite can be made through designing its proper morphology by adopting a suitable method of preparation. It is noted that binary nanocomposites, synthesized from organic–organic (like, graphene–PANI)6,7 and organic–inorganic (metal oxide–graphene/PANI)8–13 hybrids have been studied enormously compare to ternary nanocomposites (TENs, e.g. metal oxide, graphene and PANI) with further improved electrical, chemical and structural properties for advanced applications like supercapacitors, lithium ion batteries, visible light water splitting, sensors etc.2,14–19
It is known that graphene (graphene oxide/chemically converted graphene, CCG/reduced graphene oxide) can able to form functional nanocomposites with organic and inorganic materials.6–13 On the other hand, PANI can grow at very low temperature (5–10 °C) by in situ polymerization of aniline monomer on different types of surfaces such as metal oxide, graphene, ice,2,20,21 forming hierarchical nanostructures in aqueous–organic interfaces and the morphology of PANI mostly dependent upon the polarity of the solvent used in the reaction medium.22,23 This is because the existing functional groups on the surfaces can offer a large number of active sites2 for its growth through non-covalent (hydrogen bonding, π–π* stacking), electrostatic and hydrophobic interactions.23 Therefore, one can construct 3D hierarchical flower-like nanostructures in a ternary system from aqueous–organic medium if the kinetic barrier can be reduced by means of controlling the composition of a CCG hybridized metal oxide to aniline proportion.2 Among the functional metal oxide semiconductors, ZnO (direct band gap, 3.37 eV) is a potential material and hence, the synthesis of hierarchical nanoflowers with ZnO, CCG and PANI can be beneficial for both fundamental research and pioneering applications in diverse fields. However, to the best our knowledge, no report is available on the synthesis of nanocomposite having hierarchical 3D nanoflowers of TENs from ZnO, CCG and PANI by surfactant-free precursor based solution process at low temperature.
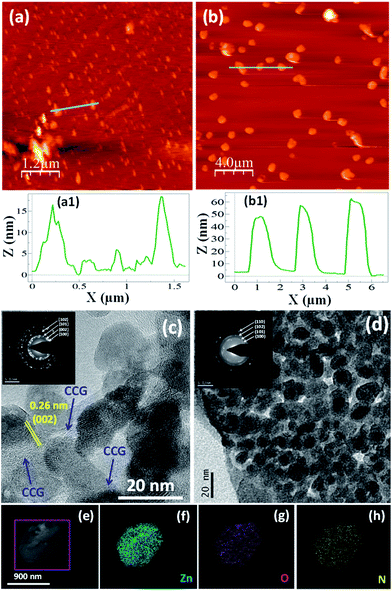
Herein, for the first time, we report the synthesis of organic–inorganic hybrid nanoflowers of ZnO–CCG–PANI (ZGP) by combining ZnO–CCG and PANI, through in situ polymerization of aniline monomer. In our previous work,13 we proposed that in the synthesis of ZnO–CCG nanocomposite by solution process, the generated ZnO/Zn2+ can react with oxygen functional groups of graphene oxide (GO), created hollow structured microspheres (Fig. 1a and b) of ZnO–CCG (ZG) nanocomposite. In this present work, the conversion of GO to CCG is confirmed from XPS, FTIR and Raman spectral studies (see ESI, Fig. S1†). On introduction of aniline monomer into the dispersion of ZG nanocomposite, three dimensional flower-like architecture is found to develop during in situ polymerization of aniline at low temperature (5–10 °C). In this ZGP nanocomposite, ZnO–CCG nanoparticles are observed to well decorate onto PANI nanosheets forming ZGP nanopetals (thickness, 50 to 70 nm) which further arranged into nanoflowers (ESI, Fig. S2†) by self-assembly.1
The hierarchical nanoflowers (average size, 3–4 μm) (Fig. 1c–f) that can have high surface to volume ratio1 as revealed by FESEM and TEM analyses. The TEM study also confirms the formation of nanoflowers (Fig. 1g) by aggregation of ZGP nanopetals. It is noted that the higher magnification TEM images clearly demonstrate the nanoparticles of ZG that are well decorated on the nanosheets of PANI. The TEM result is also supported by AFM analysis of ZG and ZGP nanocomposites (Fig. 2a and b) as the AFM height profile of the ZGP nanocomposite is found to be higher in magnitude compare to that of ZG. This is because the nanostructured petals generated from the inorganic–organic nanohybrid can result an increase in thickness of ZGP nanocomposite particles due to the presence of PANI layers on ZG particle surface. Fig. 2c presents the TEM image of ZG nanocomposite where ZnO nanoparticles are chemically interacted with graphene layers via the oxygen functional groups of CCG.13 In fact, the particle size of ZGP is higher compare to ZG. This can also imply the formation of PANI layer onto the surface of ZG nanoparticles (Fig. 2d). The TEM-SAEDs of ZG and ZGP (inset, Fig. 2c and d) confirm the presence of hexagonal-ZnO (h-ZnO) and the XRD patterns of ZGP sample (see ESI, Fig. S3†) fully support the TEM result. In addition, the TEM elemental mapping as shown in Fig. 2e–h also evidences the presence of ZnO NPs well decorated on the nanosheets of PANI. It is worthy to note that the interaction of PANI with the ZG in ZGP sample is well supported by XPS, FTIR and Raman spectral analyses (see ESI, Fig. S1†).
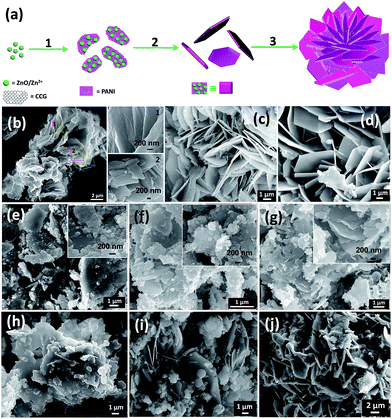
On the basis of change in morphology of ZGP due to variation of the reaction time, we propose the formation mechanism of hierarchical nanoflowers (Fig. 3a) of ZnO–CCG–PANI nanocomposite. It is noted that the ZnO–CCG (ZG) nanocomposite can form by the interaction of ZnO/Zn2+ moiety13 with the available oxygen functional groups of the GO via layer by layer chemical exfoliation of graphene layers (Fig. 2c). At an initial stage (schematically shown in Fig. 3a), after introduction of PANI (in situ polymerization of aniline monomer) in ZG, the nanocomposite can offer numerous active sites for the 2D growth of PANI towards formation of nanosheet-like structure. However, after 2 h reaction, the growth (step 1, Fig. 3a and b) is found towards the formation of 2D morphology of ZGP where PANI is noticed to be rapped on ZG particle surface via interaction with ZnO–CCG (ZG) moiety. But in the second step (step 2, after 4 h, Fig. 3a), the kinetically controlled growth of the nanosheets can further construct the structure via aggregation of individual ZG nanoparticles to form separate petals (Fig. 3c) for minimizing the surface energy of the nanocomposite particles. In the final step (step 3, after 6 h, Fig. 3a), hopefully due to an anisotropic growth of the nanopetals1 provide complete formation of flower-like structures (Fig. 1c). Therefore, in this growth mechanism, PANI is attached to the ZG by chemical interaction of –NH groups in ZGP nanocomposite. Moreover, ZG can serve as a nucleation sites to form scaffolds of the nanopetals. On the other hand, the formation of final product is found to be dependent upon the concentration of PANI i.e. weight ratio of ZG to aniline monomer (R). It is seen that when the R is high i.e. in the cases of R, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in ZGP20 or R, 14
1 in ZGP20 or R, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in ZGP14, the ZG nanoparticles may aggregate under diffusion control mechanism,1,24–26 resulting the formation of compact structure of the ZGP nanoparticles along with a few nanosheet-like protrusions (Fig. 3e and f). However, at an optimum concentration of aniline at the R value, 7
1 in ZGP14, the ZG nanoparticles may aggregate under diffusion control mechanism,1,24–26 resulting the formation of compact structure of the ZGP nanoparticles along with a few nanosheet-like protrusions (Fig. 3e and f). However, at an optimum concentration of aniline at the R value, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ZGP), it can reduce the influence of diffusion of the particles, resulting the formation of nanoflowers (Fig. 1c–g). It is interesting to note that when the concentration of aniline is very high (low R, 3
1 (ZGP), it can reduce the influence of diffusion of the particles, resulting the formation of nanoflowers (Fig. 1c–g). It is interesting to note that when the concentration of aniline is very high (low R, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in ZGP3), a random creation of PANI protuberance of the petal nanosheets with branched structures resulted irregular-shaped petals is observed (Fig. 3g). It is further noted that not only the influence of ZG to aniline weight ratio (R) but also the water to ethanol proportion can play a vital role on the formation of ZGP nanoflowers. To understand this effect, we have synthesized the ZGP nanocomposite by separately using ethanol and double distilled water (see Experimental, ESI†) in the reaction medium. It is worthy to note that a hydrophilic emeraldine PANI salt can produce in the reaction medium. In this respect, the affinity of the salt with the solvents can decrease due to decrease in solvent polarity22 from water to ethanol. As a result, the formation of more compact structure of nanoflowers (Fig. 3h) is favored in ethanol medium (ZGPE). Moreover, it is seen that the petals is well separated with PANI nano/micro-spheres (Fig. 3i) in water medium (ZGPW). In this regard, the presence of soft templates of aniline micelles or in situ generated structure of PANI can help to form the spheres.2,22 Therefore, in the reaction medium, the water to ethanol proportion optimization is necessary for formation of the hierarchical nanoflowers. To further understand the formation mechanism of the nanoflowers, we have also synthesized the nanocomposites without using GO or ZnO (Fig. 3j and S4, ESI†) under the similar preparative conditions as done for ZGP sample. In this case, no flower-like structures are noticed in absence of ZnO or GO (Fig. 3j and S4, ESI†). However, a randomly connected PANI protuberance combined with nanosheets is observed in absence of GO (Fig. 3j). This observation implies that the presence of active nucleation sites of GO or ZnO is highly needed for the 3D growth of PANI (Fig. 3j).
1 in ZGP3), a random creation of PANI protuberance of the petal nanosheets with branched structures resulted irregular-shaped petals is observed (Fig. 3g). It is further noted that not only the influence of ZG to aniline weight ratio (R) but also the water to ethanol proportion can play a vital role on the formation of ZGP nanoflowers. To understand this effect, we have synthesized the ZGP nanocomposite by separately using ethanol and double distilled water (see Experimental, ESI†) in the reaction medium. It is worthy to note that a hydrophilic emeraldine PANI salt can produce in the reaction medium. In this respect, the affinity of the salt with the solvents can decrease due to decrease in solvent polarity22 from water to ethanol. As a result, the formation of more compact structure of nanoflowers (Fig. 3h) is favored in ethanol medium (ZGPE). Moreover, it is seen that the petals is well separated with PANI nano/micro-spheres (Fig. 3i) in water medium (ZGPW). In this regard, the presence of soft templates of aniline micelles or in situ generated structure of PANI can help to form the spheres.2,22 Therefore, in the reaction medium, the water to ethanol proportion optimization is necessary for formation of the hierarchical nanoflowers. To further understand the formation mechanism of the nanoflowers, we have also synthesized the nanocomposites without using GO or ZnO (Fig. 3j and S4, ESI†) under the similar preparative conditions as done for ZGP sample. In this case, no flower-like structures are noticed in absence of ZnO or GO (Fig. 3j and S4, ESI†). However, a randomly connected PANI protuberance combined with nanosheets is observed in absence of GO (Fig. 3j). This observation implies that the presence of active nucleation sites of GO or ZnO is highly needed for the 3D growth of PANI (Fig. 3j).
The electrical property of the ZGP nanoflowers (ZGP) is characterized by current–voltage (I–V) measurement at room temperature. The I–V curves (Fig S5, ESI†) show typical linear ohmic behaviors. On the other hand, in comparison to other samples, ZGP exhibits higher electrical current. This higher conductivity of ZGP sample can attribute to the extended conjugation of π-electrons20,21 of PANI which is grown along its various crystallographic orientations on ZnO–CCG surface. This architecture can assume to have superior electrolyte permeability in the form of three dimensional conducting network of the nanoflowers.27–30 It is noted that an inferior electrochemical activity of ZG and ZP samples is obtained compare to the ZGP nanoflowers (Fig. S6, ESI†). The inferior electrochemical activity of ZP and ZG can be attributed to the absence of the synergic effect of graphene and PANI components as well as the non-existence of the hierarchical morphology (three dimensional nanoflowers).14,31 Moreover, the nanoflowers of ZGP sample shows an increased area in the cyclic-voltammetry (CV) curve at a scan rate of 10 mV s−1, indicating a higher capacitance value of ZGP is possible than that of the other samples (ZGPE and ZGPW) (Fig. 4a). In the CV curves of ZGP, ZGPE and ZGPW samples, a pair of weak intensity redox peaks is found (Fig. 4a). Actually, PANI can exist in three different states; leucoemeraldine, polaronic emeraldine and pernigraniline.32 The pair of redox peaks of ZGP, ZGPE and ZGPW samples can be related to the inter conversion of leucoemeraldine and polaronic emeraldine states of PANI.33,34 Also, it is found that the area of the CV curve is increased with the scan rate for ZGP nanoflowers (Fig. 4b). This result can be related to the superior surface characteristic of ZGP nanoflowers. In this regard, the nanoflowers can offer conducting three dimensional longer diffusion paths for transport of electrons that may be due to the presence of nanochannels in the nanostructures.27,28,30 On the other hand, the interconnected conducting pathways of the nanoflowers can facilitate fast diffusion of electrolytes and improve the kinetics of reversible redox process for charge storage.27,28,30
 | ||
| Fig. 4 Cyclic voltammetry result of (a) ZGP, ZGPE and ZGPW samples at a fixed scan rate of 10 mV s−1 and (b) ZGP at different scan rates embedded in the figure. | ||
In summary, we have successfully synthesized three dimensional (3D) hierarchical inorganic–organic hybrid nanoflowers of ZnO–chemically converted graphene (ZG)–polyaniline (ZGP) nanocomposite from surfactant-free low temperature solution process. Construction of conducting 3D ZGP nanoflowers is seen through optimization of precursor composition (ZG to aniline weight ratio and water to ethanol proportion) and reaction time. These factors are found to be greatly influenced upon the formation of nanoflowers but the nanoparticles of ZG are observed to be well decorated on the nanopetals of polyaniline (PANI) via chemical interactions. In an optimized precursor composition by increasing the reaction time, the in situ polymerization of aniline monomer to PANI in ZG nanocomposite, the formation of 2D nanosheet-like structure of PANI along with the ZG particles well rapped with PANI is observed after 2 h reaction time. The ZGP nanosheets further construct the structure via aggregation of individual ZG nanoparticles to form separate petals after 4 h. However, at 6 h, the complete formation of flower-like 3D nanostructures is observed. The ZGP nanoflowers show enhanced electrochemical activity which may find applications in sensors, electrocatalysis and energy storage devices.
Acknowledgements
Authors wish to acknowledge the Director, CSIR-CGCRI, Kolkata, India for his kind permission to publish this work. The authors SB, AN and MP thank Council of Scientific and Industrial Research (CSIR) and UGC, Govt. of India for providing their research fellowships. Authors also acknowledge the help rendered by Nanostructured Materials Division and Electron Microscopy Section for Raman and microstructural characterizations, respectively. The work has been done as an associated research work of 12th Five Year Plan project of CSIR (No. ESC0202).Notes and references
- J. Ge, J. Lei and R. N. Zare, Nat. Nanotechnol., 2012, 7, 428–432 CrossRef CAS PubMed.
- S. Giri, D. Ghosh and C. K. Das, Adv. Funct. Mater., 2014, 24, 1312–1324 CrossRef CAS.
- A. Kaushik, R. Kumar, S. K. Arya, M. Nair, B. D. Malhotra and S. Bhansali, Chem. Rev., 2015, 115, 4571–4606 CrossRef CAS PubMed.
- Z. Lin, Y. Xiao, Y. Yin, W. Hu, W. Liu and H. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 10775–10782 CAS.
- Z. Wu, Y. Wu, T. Pei, H. Wang and B. Geng, Nanoscale, 2014, 6, 2738–2745 RSC.
- Y. Meng, K. Wang, Y. Zhang and Z. Wei, Adv. Mater., 2013, 25, 6985–6990 CrossRef CAS PubMed.
- J. Xu, K. Wang, S.-Z. Zu, B.-H. Han and Z. Wei, ACS Nano, 2010, 4, 5019–5026 CrossRef CAS PubMed.
- X. Li, X. Huang, D. Liu, X. Wang, S. Song, L. Zhou and H. Zhang, J. Phys. Chem. C, 2011, 115, 21567–21573 CAS.
- S. Bera, N. Das, M. Pal, S. Mahanty and S. Jana, J. Sol-Gel Sci. Technol., 2015, 76, 402–413 CrossRef CAS.
- N. Guo, Y. Liang, S. Lan, L. Liu, J. Zhang, G. Ji and S. Gan, J. Phys. Chem. C, 2014, 118, 18343–18355 CAS.
- H. Zhang, R. Zong and Y. Zhu, J. Phys. Chem. C, 2009, 113, 4605–4611 CAS.
- C. Janaky, N. R. de Tacconi, W. Chanmanee and K. Rajeshwar, J. Phys. Chem. C, 2012, 116, 4234–4242 CAS.
- S. Bera, M. Pal, A. Naskar and S. Jana, J. Alloys Compd., 2016, 669, 177–186 CrossRef CAS.
- X. Xia, Q. Hao, W. Lei, W. Wang, D. Sun and X. Wan, J. Mater. Chem., 2012, 22, 16844–16850 RSC.
- G. Han, Y. Liu, L. Zhang, E. Kan, S. Zhang, J. Tang and W. Tang, Sci. Rep., 2014, 4, 4824 Search PubMed.
- Y. Dong, Z. Zhao, Z. Wang, Y. Liu, X. Wang and J. Qiu, ACS Appl. Mater. Interfaces, 2015, 7, 2444–2451 CAS.
- F. Zhang, H. Cao, D. Yue, J. Zhang and M. Qu, Inorg. Chem., 2012, 51, 9544–9551 CrossRef CAS PubMed.
- L. Jing, Z.-Y. Yang, Y.-F. Zhao, Y.-X. Zhang, X. Guo, Y.-M. Yan and K. N. Sun, J. Mater. Chem. A, 2014, 2, 1068–1075 CAS.
- Z. Ye, Y. Jiang, H. Tai, N. Guo, G. Xie and Z. Yuan, J. Mater. Sci.: Mater. Electron., 2015, 26, 833–841 CrossRef CAS.
- Y. Wang, J. A. Torres, A. Z. Stieg, S. Jiang, M. T. Yeung, Y. Rubin, S. Chaudhuri, X. Duan and R. B. Kaner, ACS Nano, 2015, 9, 9486–9496 CrossRef CAS PubMed.
- I. Y. Choi, J. Lee, H. Ahn, J. Lee, H. C. Choi and M. J. Park, Angew. Chem., Int. Ed., 2015, 54, 10497–10501 CrossRef CAS PubMed.
- J. Huang and R. B. Kaner, Angew. Chem., Int. Ed., 2004, 43, 5817–5821 CrossRef CAS PubMed.
- Y. Wang, J. Liu, H. D. Tran, M. Mecklenburg, X. N. Guan, A. Z. Stieg, B. C. Regan, D. C. Martin and R. B. Kaner, J. Am. Chem. Soc., 2012, 134, 9251–9262 CrossRef CAS PubMed.
- T. A. Witten and L. M. Sander, Phys. Rev. Lett., 1981, 47, 1400–1402 CrossRef CAS.
- J. Zeng and Y. Xia, Nat. Nanotechnol., 2012, 7, 415–416 CrossRef CAS PubMed.
- B. Ahmad, Y. Chen and L. J. Lapidus, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 2336–2341 CrossRef CAS PubMed.
- L. Li, J. Ma, Z. Zhang, B. Cao, Y. Wang, L. Jiao and H. Yuan, ACS Appl. Mater. Interfaces, 2015, 7, 23978–23983 CAS.
- S.-I. Kim, J.-S. Lee, H.-J. Ahn, H.-K. Song and J.-H. Jang, ACS Appl. Mater. Interfaces, 2013, 5, 1596–1603 CAS.
- S. Bhattacharya, U. Rana and S. Malik, J. Phys. Chem. C, 2013, 117, 22029–22040 CAS.
- B. Yang, J. Wang, D. Bin, M. Zhu, P. Yanga and Y. Du, J. Mater. Chem. B, 2015, 3, 7440–7448 RSC.
- X. Xia, Q. Hao, W. Lei, W. Wang, H. Wang and X. Wang, J. Mater. Chem., 2012, 22, 8314–8320 RSC.
- X.-M. Feng, R.-M. Li, Y.-W. Ma, R.-F. Chen, N.-E. Shi, Q.-L. Fan and W. Huang, Adv. Funct. Mater., 2011, 21, 2989–2996 CrossRef CAS.
- Y. Liu, Y. Ma, S. Guang, H. Xu and X. Su, J. Mater. Chem. A, 2014, 2, 813–823 CAS.
- Z. Zhang, Y. Zhang, K. Yang, K. Yi, Z. Zhou, A. Huang, K. Mai and X. Lu, J. Mater. Chem. A, 2015, 3, 1884–1889 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials synthesis and characterizations with XPS, FTIR, Raman, FESEM, XRD, I–V and CV results of different samples. See DOI: 10.1039/c6ra05698a |
| This journal is © The Royal Society of Chemistry 2016 |