Self-assembly of isomannide-based monoesters of C18-fatty acids and their cellular uptake studies†
Prabhu Dhasaiyanab,
Nimisha Parekhc,
T. Vijai Kumar Reddyd,
G. Sandhya Ranid,
B. L. A. Prabhavathi Devi*d and
B. L. V. Prasad*ab
aPhysical and Materials Chemistry Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune – 411008, India. E-mail: pl.bhagavatula@ncl.res.in; Fax: +91 20 25902636; Tel: +91 20 25902013
bAcademy of Scientific and Innovative Research (AcSIR), New Delhi 110 025, India
cChemical Engineering and Process Development Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune – 411008, India
dCentre for Lipid Research, CSIR-Indian Institute of Chemical Technology, Hyderabad – 500007, India. E-mail: prabhavathi@iict.res.in; Fax: +91 40 27193370; Tel: +91 40 27191845
First published on 19th July 2016
Abstract
The self-assembling behavior of oleic, elaidic and stearic acid-isomannide glycolipids is revealed. Amongst these, oleic and elaidic acid-based isomannide lipids self-assembled to form microspheres which were efficiently taken up by cancer cell lines enabling their usage for drug delivery applications.
1. Introduction
Self-assembly connotes spontaneous organization of building blocks into ordered structures and is ubiquitous in living systems.1 Self-assembled structures have a wide range of applications that span from biology to materials science.2 Among the different classes of molecules such as dendrimers,3 polymers,4 peptides,5 OPVs,6 foldamers,7 etc. that are being studied for their self-assembling characteristics, glycolipids are the recent entries.8 The structural variations offered by them, which include multiple hydrogen bonding, van der Waals interaction etc., have prompted researchers to investigate their self-assembling behavior with great vigor. Glycolipids are an important class of amphiphiles which control many biological functions like cell adhesion, recognition, signaling and differentiation, maintenance of membrane integrity, photosynthesis, binding and transfection of viruses.9 Under suitable condition they form different self-assembled structures including lamellar, hexagonal or cubic phases.10 The head group structure, (configuration of the anomeric carbon) as well as the number of sugar residues also influences their phase behaviour.11 However, it must be noted that the self-assembling characteristics of glycolipids are much less understood as compared to those of phospholipids.12 Isomannide is a double dehydrated product of mannitol, which itself is obtained as a by-product in starch industry. Isomannides are being used extensively in areas like polymer synthesis, preparation of chiral ionic liquids etc.13,14 Isomannide is a rigid moiety and was found to be non-toxic. For these reasons isomannides are extensively used in the pharmaceutical industry. For example, Puzer et al. reported the human kallikrein 5 (KLK5) and 7 (KLK7) inhibition of isomannide based peptidomimetic compounds and which are potent even at low micromolar range.15 Considering the myriad biological and other applications listed above we envisaged that self-assembled structures based on isomannide-glycolipids could become interesting candidates for drug delivery applications as they provide greater control over the structures being formed thus leading to formulations with improved performance. Accordingly, we embarked on a study of the self-assembling properties of isomannide attached to different fatty acids (oleic, elaidic and stearic acid). The specific isomannide-based lipids (IMLs) used in this study are isomannide monooleate (OAIML), isomannide monoelaidiate (EAIML) and isomannide monostearate (SAIML).2. Experimental section
A. Materials
The fatty acids namely oleic acid, elaidic acid, stearic acid and the dye molecule fluorescein were purchased from Sigma Aldrich and used as received without any further purification. Milli Q purified water with a resistivity of 18.2 MΩ cm was used throughout the experiments. The mannitol used was purchased from M/s. SD Fine Chemicals Pvt. Ltd., Mumbai, India. FBS (Fetal Bovine Serum), DMEM (Dulbecco's Modified Eagle's medium), MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) were purchased from Invitrogen, India. MDA-MB-231, triple negative breast cancer cell line was purchased from National Centre For Cell Science (NCCS), Pune, Maharashtra, India. All solvents used for the experiments are analytical grade (AR).B. Synthesis of different IMLs
Pure isomannide-based monoesters of oleic, elaidic and stearic acids were synthesized following procedures reported for oleic and stearic IMLs.16 The detailed synthetic procedure can be found in ESI-1.† Though the spectral details of OAIML and SAIML have already been reported, for the sake of comparison the characterization details of all the compounds have been provided [please see ESI-2, Fig. S1(a1–c4)†].C. Preparation of self-assembled structures
The self-assemblies of these lipids were accessed by adding calculated amount of OAIML, EAIML and SAIML into 1 mL of Milli Q water. In case of OAIML and EAIML either 500 μg or 1 mg of the compounds were dissolved in 1 mL of Milli Q water where as for SAIML 600 μg of compound was dissolved in 1 mL of Milli Q water. These were then sonicated using a sonication bath for a maximum of 3 minutes until clear solution formed. The sonication was done in Equitron (Digital ultrasonic cleaner) with 2.5 L capacity and heater facility. Please note that the sample was not heated to prepare self-assembled structure in any cases. These clear solutions were left untouched and after a specific amount of period the clear solutions transformed into cloudy one (for detailed preparation procedure see ESI-3†). This was taken as an indication for the formation of self-assembled structures. In order to visualize the structures formed, optical microscopy (OM), Scanning Electron Microscopy (SEM), Confocal Laser Scanning Microscopy (CLSM) and Atomic Force Microscopy (AFM) were employed. Amongst these while the OM experiments were done using the solutions directly avoiding drying, for all the others a measured quantity of the solution was drop casted on an appropriate substrates and the analysis was carried out on these dried samples (vide infra).D. Instrumentation and characterization of supramolecular structures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2SO4 and 30% H2O2) was used to prepare dustless glass slides and cover slips. The self-assembled structures were viewed using LEICA DM2500 P-polarized light microscope with 4×, 10×, and 20× objectives, fitted with a heating stage. The images were recorded by using LEICA DFC 500 camera.
1 H2SO4 and 30% H2O2) was used to prepare dustless glass slides and cover slips. The self-assembled structures were viewed using LEICA DM2500 P-polarized light microscope with 4×, 10×, and 20× objectives, fitted with a heating stage. The images were recorded by using LEICA DFC 500 camera.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (by volume) ratio taken in a dialysis membrane (which was stored in sodium azide solution having a concentration of 0.05% and purchased from Spectrum Labs.Com) with a molecular weight cut-off of 2000 Da. The bag was sealed off at the ends and the sample was subjected to dialysis against de-ionized water for 24 h. Fresh Milli Q water solution was added at certain intervals of time until the outside solution remained colourless to ensure the removal of un-encapsulated dye.
2 (by volume) ratio taken in a dialysis membrane (which was stored in sodium azide solution having a concentration of 0.05% and purchased from Spectrum Labs.Com) with a molecular weight cut-off of 2000 Da. The bag was sealed off at the ends and the sample was subjected to dialysis against de-ionized water for 24 h. Fresh Milli Q water solution was added at certain intervals of time until the outside solution remained colourless to ensure the removal of un-encapsulated dye.| Relative percent cell viability = Atest/Acontrol × 100% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well in DMEM containing 10% FBS. The plate containing the cells was incubated at 37 °C with 5% CO2 for 24 h. Now the DMEM media was replaced with 80 μg mL−1 of fluorescein encapsulated IML microspheres and incubation continued for 4 h under the above mentioned conditions. Once the incubation was complete, cells were washed three times with PBS and fixed with 4% paraformaldehyde. Nucleus was stained with DAPI after adding fluorescein encapsulated IMLs.
000 cells per well in DMEM containing 10% FBS. The plate containing the cells was incubated at 37 °C with 5% CO2 for 24 h. Now the DMEM media was replaced with 80 μg mL−1 of fluorescein encapsulated IML microspheres and incubation continued for 4 h under the above mentioned conditions. Once the incubation was complete, cells were washed three times with PBS and fixed with 4% paraformaldehyde. Nucleus was stained with DAPI after adding fluorescein encapsulated IMLs.3. Results and discussion
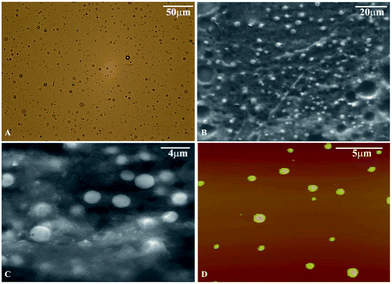
Our investigation started with the preparation of pure form of different IMLs. The chemical structures of the C18-fatty acid based isomannide lipids (IMLs) employed in this study are shown in Fig. 1. Before proceeding to the investigations on the self-assembled structure formation with these molecules, the critical micelle concentration (CMC) of both OAIMLs and EAIMLs was determined using pyrene as a fluorescence probe. The cmc values of OAIML and EAIML are found to be 5.6 × 10−6 M and 3.16 × 10−6 M respectively (please see ESI-5, Fig. S3†).These molecules were then dissolved in water at 500 μg mL−1 or 1 mg mL−1 concentrations with the help of sonication. The clear solutions thus formed when left untouched for sometime started becoming hazy indicating the formation of self-assembled structures. We wish to note here that while the formation of spherical structures at 500 μg mL−1 was confirmed based on optical (please see ESI-6, Fig. S4†) and confocal microscope analysis with OAIML and EAIML (please see ESI-7, Fig. S5†), the number of such structures was few. Gratifyingly at 1 mg mL−1 concentrations more number of spherical self-assembled structures could be clearly seen. To check the stability of the self-assembled structures at elevated temperatures, we heated the self-assembled structures to 40 °C and the samples were imaged using OM. The results suggest that the spherical structure remain intact without any disruption (please see ESI-8, Fig. S6†). The average diameter of the spherical structures was estimated to be 5–6 μm for OAIML and 2–5 μm for EAIML (Fig. 2A and 3A). The formation of spherical structures is confirmed from other microscopic imaging techniques like SEM and AFM also (Fig. 2B–D for OAIML and Fig. 3B–D for EAIML). The sizes of the structures determined from SEM and AFM analyses are different when compared to those obtained from OM images. This could be ascribed to the fact that the latter two analyses are carried out on dried samples which can lead to aggregation of structures, whereas optical microscopy is carried out with the solvent. The uniformity of dye encapsulation as revealed by CLSM analysis (ESI-7, Fig. S5†) confirms these structures to be consisting of solid spheres. SAIMLs, on the other hand, were found to assemble into sheet-like structures (Fig. 4).
 | ||
| Fig. 2 (A) Optical microscope images, (B) and (C) SEM images and (D) AFM images of self-assembled structures of OAIMLs. | ||
 | ||
| Fig. 3 (A) Optical microscope image, (B) and (C) SEM images and (D) AFM images of self-assembled structures of EAIMLs. | ||
 | ||
| Fig. 4 (A) Optical microscope image, (B) and (C) SEM images and (D) AFM images of self-assembled structures of SAIMLs. | ||
The self-assembled structures of glycoamphiphiles are mainly used in the area of bio-imaging, drug delivery, and other bio related applications.17 Also in literature, it has been shown that microspheres can be used as drug carriers by encapsulating drug molecules in them.18 As discussed earlier, since isomannides are being used in pharma field, we wanted to evaluate the efficacy of the OAIML and EAIML microspheres as delivery vehicles by investigating their cellular uptake characteristics. Since SAIMLs formed sheet like structures they were not considered for cellular uptake experiments.
Prior to the cellular uptake experiments, the self-assembled structures of OAIML and EAIML, were loaded with fluorescein dye (as a model drug) by incubating the dye with the self-assembled IMLs (please see Experimental section for details). These were then analyzed with UV-Vis spectroscopy based on which (for complete details please see ESI-9, Fig. S7†) the amount of dye encapsulated in the self-assembled microspheres was determined to be 0.10 μg and 0.28 μg for EAIML and OAIML microspheres respectively. A control sample was made by taking 1 mL of 10 μM dye in DMEM media. This corresponds to 1.56 μg of free dye per mL.
These samples were then used for the cellular uptake measurements which were performed by incubating MDA-MB-231 cells with 80 μg mL−1 of cargo (fluorescein) encapsulated EAIMLs or OAIMLs. Fig. 5 shows the epifluorescence and DIC images of cells which clearly confirms that the self-assembled structures of EAIMLs and OAIMLs are able to deliver the fluorescein into the cells very effectively. The overlay of fluorescence and DIC images discloses that the emission from fluorescein (for the emission spectra of free and encapsulated fluorescein please see ESI-10, Fig. S8†) is confined to the peri-nuclear region of the cytosol (Fig. 5C, G, and K). Based on the quantitative analysis, the intensity of the emission when cells were incubated with dye loaded EAIML microspheres was determined to be 245, whereas the same for OAIML was found to be 257. Very interestingly the emission intensity when the cells were incubated with free dye was found to be lower (163) even though the cells were treated with large amount of free dye (please see ESI-11, Fig. S9†). These results imply that, although the cells were incubated with high amount of free dye, the self-assembled microsphere have delivered more dye into the cells. Based on the difference in the amount of dye used and the enhanced fluorescence intensity, it can be estimated that the dye encapsulated by IMLs exhibit 9 times (OAIML) or 22 times (EAIML) increased cargo carrying efficiency. We believe that the rigidity of isomannide group and the formation of spherical self-assembled structures help to penetrate the cell wall in order to release the cargo in an efficient way. The small amount of dye internalization with the free drug (Fig. 5C) on the other hand could be attributed to the negative charge of the dye that helps its uptake by the cells either by diffusion or pinocytosis. On the other hand, the enhanced dye uptake with OAIML or EAIML spheres could be attributed to the endocytic mechanism through which such spherical particles are known to be internalized by the cells.
 | ||
| Fig. 5 Epifluorescence images (A–D) of free cells treated with 1.56 μg of free dye, (E–H) and (I–L) images of MDA-MB-231 cells incubated with fluorescein loaded EAIMLs and OAIMLs. | ||
Finally to check the bio-compatibility of the OAIMLs and EAIMLs, the cell viability assay was performed using MTT assay.19 The viability of untreated MDA-MB-231 cells is assumed to be 100%. The cell viability was calculated as a percentage relative to untreated control cells. Polyethylenimine (PEI) was used as a negative control. The MTT assay reveals that 80% cell survival with a concentration up to 80 μg mL−1 (for more information please see ESI-12, Fig. S10†). The plot of percentage of cell viability against the concentration of IMLs is shown in Fig. S11 (please see ESI-13†). Based on the MTT assay, OAIMLs showed 90% viability even up to 500 μg mL−1 and on the other hand EAIMLs showed the same up to 200 μg mL−1. It is worth noting here that at 80 μg mL−1 (where the cellular uptake experiments were carried out) both OAIML and EAIML display appreciably low toxicity towards cell proliferation indicating good biocompatibility of these microspheres and their applicability for drug delivery application.
4. Conclusions
In conclusion, we have studied the self-assembling behaviour of isomannide based glycolipids which differ only in their hydrophobic core. The self-assemblies of elaidic acid and oleic acid IMLs resulted in the formation of microspheres which were used in cellular uptake studies using MDA-MB-231 cells. The results suggest that, the self-assembled structures show an increase in the uptake of model hydrophilic drug called fluorescein. Thus these materials can be used in drug carrier and other bio related applications.Acknowledgements
P. D, G. S. R and T. V. K. R thank UGC and CSIR (Govt. of India), New Delhi for Senior Research Fellowships respectively. We acknowledge Dr M. Jayakannan (IISER, Pune) for allowing us to avail the optical microscope facilities. We thank Mr Ketan Bhotkar and Mr Puneet Khandelwal for their technical assistance with SEM and AFM, respectively. N. P. expresses sincere acknowledgement to M2D (CSC0134) project for the financial support. We also extend our thanks to CIF, SPP University for the confocal experiments.Notes and references
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418 CrossRef CAS PubMed; G. M. Whitesides and M. Boncheva, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4769 CrossRef PubMed.
- Z. Nie, A. Petukhova and E. Kumacheva, Nat. Nanotechnol., 2010, 5, 15 CrossRef CAS PubMed; R. Verduzco, X. Li, S. L. Pesek and G. E. Stein, Chem. Soc. Rev., 2015, 44, 2405 RSC.
- E. Gazit, Nat. Nanotechnol., 2008, 3, 8 CrossRef CAS PubMed; T. Zhou, P. Chen, L. Niu, J. Jin, D. Liang, Z. Li, Z. Yang and D. Liu, Angew. Chem., 2012, 51, 11271 CrossRef PubMed; R. Mezzenga, J. Ruokolainen, N. Canilho, E. Kasemi, D. A. Schluter, W. B. Lee and G. H. Fredrickson, Soft Matter, 2009, 5, 92 RSC.
- O. Lkkala and G. ten Brinke, Science, 2002, 295, 2407 CrossRef PubMed.
- J. D. Hartgerink, E. Beniash and S. I. Stupp, Science, 2001, 294, 1684 CrossRef CAS PubMed.
- C. C. Lee, C. Grenier, E. W. Meijer and A. P. H. J. Schenning, Chem. Soc. Rev., 2009, 38, 671 RSC; S. S. Babu, V. K. Praveen, K. K. Kartha, S. Mahesh and A. Ajayaghosh, Chem.–Asian J., 2014, 9, 1830 CrossRef CAS PubMed; Y. Hirai, S. S. Babu, V. K. Praveen, T. Yasuda, A. Ajayaghosh and T. Kato, Adv. Mater., 2009, 21, 4029 CrossRef; V. K. Praveen, C. Ranjith, E. Bandini, A. Ajayaghosh and N. Armaroli, Chem. Soc. Rev., 2014, 43, 4222 RSC; J. F. Hulvat, M. Sofos, K. Tajima and S. I. Stupp, J. Am. Chem. Soc., 2005, 127, 366 CrossRef PubMed.
- D. S. Garcia, B. Kauffmann, T. Kawanami, H. Ihara, M. Takafuji, M. H. Delville and I. Huc, J. Am. Chem. Soc., 2009, 131, 8642 CrossRef CAS PubMed; Q. Gan, Y. Ferrand, C. Bao, B. Kauffmann, A. Grelard, H. Jiang and I. Huc, Science, 2011, 331, 1172 CrossRef PubMed; M. Kudo, V. Maurizot, B. Kauffmann, A. Tanatani and I. Huc, J. Am. Chem. Soc., 2013, 135, 9628 CrossRef PubMed; N. Chandramouli, Y. Ferrand, G. Lautrette, B. Kauffmann, C. D. Mackereth, M. Laguerre, D. Dubreuil and I. Huc, Nat. Chem., 2015, 7, 334 CrossRef PubMed.
- P. Dhasaiyan, A. Banerjee, N. Visaveliya and B. L. V. Prasad, Chem.–Asian J., 2013, 8, 369 CrossRef CAS PubMed; N. Baccile, J. K. Pederson, G. P. Arnaudet and I. N. A. V. Bogaert, Soft Matter, 2013, 9, 4911 RSC; N. Baccile, F. Babonneau, J. Jestin, G. P. Arnaudet and I. N. A. V. Bogaert, ACS Nano, 2012, 6, 4763 CrossRef PubMed; P. Dhasaiyan, P. R. Pandey, N. Visaveliya, S. Roy and B. L. V. Prasad, Chem.–Eur. J., 2014, 20, 6246 CrossRef PubMed; A. S. Cuvier, J. Berton, C. V. Stevens, G. C. Fadda, F. Babonneau, I. N. A. Van Bogaert, W. Soetaert, G. P. Arnaudet and N. Baccile, Soft Matter, 2014, 10, 3950 RSC; A. S. Cuvier, F. Babonneau, J. Berton, C. V. Stevens, G. C. Fadda, I. Genois, P. Le Griel, G. P. Arnaudet and N. Baccile, Chem.–Asian J., 2015, 10, 2419 CrossRef PubMed; P. R. Pandey, P. Dhasaiyan, B. L. V. Prasad and S. Roy, Z. Phys. Chem., 2016, 230, 819 CrossRef.
- K. Kasahara and Y. Sanai, Glycoconjugate J., 2000, 17, 153 CrossRef CAS PubMed; A. R. Todeschini and S. I. Hakomori, Biochim. Biophys. Acta, 2008, 1780, 421 CrossRef PubMed; A. R. Wellburn, New Phytol., 1997, 135, 115 Search PubMed; A. Minoda, N. Sato, H. Nozaki, K. Okada, H. Takahashi, K. Sonoike and M. Tsuzuki, Eur. J. Biochem., 2002, 269, 2353 CrossRef PubMed; K. Nakata, C. T. Guo, M. Matsufuji, A. Yoshimoto, M. Inagaki, R. Higuchi and Y. Suzuki, J. Biochem., 2000, 127, 191 CrossRef PubMed.
- M. Corti, L. Cantu, P. Brocca and E. Del Favero, Curr. Opin. Colloid Interface Sci., 2007, 12, 148 CrossRef CAS.
- M. Hato, Curr. Opin. Colloid Interface Sci., 2001, 6, 268 CrossRef CAS.
- U. M. Migas, L. Abbey, T. V. Torrijos and J. J. McManus, Soft Matter, 2014, 10, 3978 RSC.
- V. Kumar, C. Pei, C. E. Olsen, S. J. C. Schaffer, V. S. Parmer and S. V. Malhotra, Tetrahedron: Asymmetry, 2008, 19, 664 CrossRef CAS.
- S. Luo, X. Mi, L. Zhang, S. Liu, H. Xu and J. P. Cheng, Angew. Chem., Int. Ed., 2006, 45, 3093 CrossRef CAS PubMed; S. A. A. Rizvi and S. A. Shamsi, Anal. Chem., 2006, 78, 7061 CrossRef PubMed; P. Wasserscheid, A. Bosmann and C. Bolm, Chem. Commun., 2002, 200–201 RSC.
- J. P. C. Oliveira, R. F. Freitas, L. S. de Melo, T. G. Barros, J. A. N. Santos, M. A. Juliano, S. Pinheiro, M. Blaber, L. Juliano, E. M. F. Muri and L. Puzer, ACS Med. Chem. Lett., 2014, 5, 128 CrossRef CAS PubMed.
- T. V. K. Reddy, G. S. Rani, R. B. N. Prasad and B. L. A. Prabhavathi Devi, RSC Adv., 2015, 5, 40997 RSC.
- K. Dan and S. Ghosh, Macromol. Rapid Commun., 2012, 33, 127 CrossRef CAS PubMed; A. Y. Shaikh, S. Das, D. Pati, V. Dhaware, S. Sengupta and S. Hotha, Biomacromolecules, 2014, 15, 3679 CrossRef PubMed.
- T. Delmas, A. Fraichard, P. A. Bayle, I. Texier, M. Bardet, J. Baudry, J. Bibette and A. C Couffin, J. Colloid Sci. Biotechnol., 2012, 1, 16 CrossRef CAS; M. D. Joshi and R. H. Muller, Eur. J. Pharm. Biopharm., 2009, 71, 161 CrossRef PubMed; W. Mehnert and K. Mader, Adv. Drug Delivery Rev., 2001, 47, 165 CrossRef PubMed; R. H. Muller, K. Mader and S. H. Gohla, Eur. J. Pharm. Biopharm., 2000, 50, 161 CrossRef PubMed.
- N. Kalva, N. Parekh and A. V. Ambade, Polym. Chem., 2015, 6, 6826 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Confocal images of fluorescein encapsulated self-assembled structures. See DOI: 10.1039/c6ra05608c |
| This journal is © The Royal Society of Chemistry 2016 |

