Fabrication of durable anticorrosion superhydrophobic surfaces on aluminum substrates via a facile one-step electrodeposition approach
Abstract
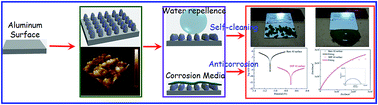
With unique water-repellent and self-cleaning properties, engineering metallic materials with superhydrophobicity endows them with greatly enhanced corrosion resistance. Herein, a facile and controllable one-step electrodeposition approach was employed to fabricate a superhydrophobic surface (SHPS) on an aluminum (Al) substrate as a barrier against corrosion media. The wettability, morphology, and chemical composition of the consequent SHPS were characterized by contact angle (CA), field-emission scanning electron microscopy (FE-SEM), atomic force microscopy (AFM), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and energy dispersive spectroscopy (EDS), respectively. The anti-contamination and anticorrosion behaviors of the resultant SHPS were investigated by self-cleaning test, potentiodynamic polarization, and electrochemical impedance spectroscopy (EIS). The electrochemical results indicate that the resultant SHPS possessed greatly enhanced corrosion resistance, and were able to reduce the corrosion of a bare Al surface, with an inhibition efficiency of 99.96%. Furthermore, the as-fabricated SHPS maintained excellent durability and stability after air exposure, deionized water immersion, and 3.5 wt% NaCl solution immersion. We believe that SHPS fabricated by a versatile one-step electrodeposition approach would make it possible to develop engineering materials with durable self-cleaning and anticorrosion properties for use in rugged environments.

 Please wait while we load your content...
Please wait while we load your content...