Stabilization of Eu3+ under a reductive atmosphere by the Al3+ co-doping of Sr2SiO4:Eu2+/Eu3+
Abstract
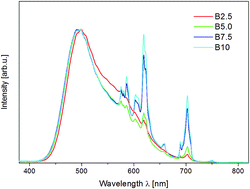
Two series of materials based on a strontium orthosilicate matrix doped with europium and different concentrations of aluminum ions were obtained using different solid state synthesis strategies. The phase composition of the samples was investigated using the X-ray diffraction (XRD) technique. The results of the optical characterization indicated that the incorporation of aluminum ions causes the stabilization of the Eu3+ ions under reductive atmosphere. The concentration ratio of europium ions incorporated into the matrix in both oxidation states ([Eu3+]/[Eu2+]) can be controlled by changing the Al3+ concentration. The observed effect is interesting from the point of view of the design of phosphors used to obtain white light emitting diodes (WLEDs). The influence of temperature on the relative intensity of Eu2+ and Eu3+ emission bands was studied to investigate the range of the impact of the created compensation defects. It is shown that the Eu3+ to Eu2+ reduction process in Sr2SiO4 takes place due to the migration of the strontium vacancies to the surface.

 Please wait while we load your content...
Please wait while we load your content...