Enhancement of anti-tumor immune responses induced by ligand-mediated biomimetic Texosomes
Zhongyan Wang,
Shasha Chang,
Xiuli Zhao,
Dawei Chen and
Kexin Li*
School of Pharmacy, Shenyang Pharmaceutical University, Liaoning Province, China. E-mail: kexinbest1128@163.com; Tel: +8602423986293
First published on 27th May 2016
Abstract
In order to overcome the immune tolerance and awake the tumor patients’ self immune response, hTERT-HSP70 as a universal tumor antigen is specifically transferred to dendritic cells by DEC205McAb Texosomes as the carriers of a tumor vaccine. The strength of the immune response and the underlying mechanisms of inhibiting tumor growth are investigated here. Our results indicate that enhanced tumor specific immune responses elicited by DEC205McAb Texosomes might be attributed to the fact that dendritic cells have a more obviously phagocytic capacity on DEC205McAb Texosomes, and thereby promote internalizing, processing and presenting of antigens as well as trigger strong tumor-specific cytotoxic T lymphocyte reaction in lymph nodes, which consequently inhibit the tumor growth and prolong the survival period. In conclusion, tumor antigen loaded ligand-mediated biomimetic Texosomes targeting dendritic cells can overcome tumor immuno-suppression and enhance the anti-tumor immune response.
Introduction
A tumor is the result of immune escape, and the body’s immune tolerance and suppression promotes tumor growth and metastasis. How to activate the tumor patients’ immune response and reduce the possibility of immune surveillance is the main target of modern cancer treatment.1 Dendritic cell (DC) immunotherapy has proven to be effective clinically for the treatment of various kinds of cancer.2–4 But at present, DCs’ adoptive immunotherapy has an individual specificity and is problematic for large-scale clinical application. In addition, a potential hazard is that an excess transfusion of DCs leads to T cell depletion and down-regulation of immune function. So how to re-awaken the dendritic cells to identify and uptake the tumor antigen in order to activate efficient anti-tumor immune response is the crucial problem that attracts our attention.Human telomerase reverse transcriptase (hTERT) plays a critical role in the malignant transformation of tumor cells.5 More than 90% of tumor cells express hTERT but normal cells don’t, so here we choose hTERT as a valuable universal antigen for a tumor vaccine.6 At the same time, heat shock protein 70 (HSP70) as an immune adjuvant is bonded with hTERT to promote internalizing, processing and presenting of antigens with major histocompatibility complex molecules (MHC) at the surface of dendritic cells through the endogenous pathway.7,8 And now, how to make DCs identify and uptake hTERT-HSP70 is an urgent problem to be solved.9,10 Texosomes are secreted from tumor cells and can transfer tumor information to dendritic cells.11,12 However, in recent years, Texosomes were found to express not only immune stimulating but also immune suppressive molecules, thereby immunogenicity was reduced.13 So in this research, we attempt to overcome the immune inhibition by biomimetic Texosomes by only carrying immune stimulating molecules hTERT-HSP70 and in the meantime DEC205 monoclonal antibody is modified on the surface of the carriers to increase the specificity to dendritic cells. We hope that ligand-coupled biomimetic Texosomes loaded with hTERT-HSP70 could efficiently be uptaken and presented by DCs, in addition triggering a stronger specific cytotoxic T lymphocyte (CTL) reaction and finally specific kill tumor cells.14
Experimental
Materials
Phosphatidylcholine (PC) was purchased from Avanti Polar Lipids (Shanghai, China), cholesterol (CH) was purchased from Tianjin Bodi Chemical (China), dioleoyl phosphoethanolamine (DOPE), 3-(N-(N,N-dimethylaminoethane)carbamoyl) cholesterol (DC Chol), 1-palmitoyl-2-[1,2-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-sn-glycero-3-phosphoethanolamine powder (NBD-PE) and DEC205 monoclonal antibody (DEC205McAb) were obtained from Sigma (UK), cholesterol succinate (CHS) was self-prepared in our lab, Cremophor EL was obtained from BASF (Germany), human telomerase reverse transcriptase peptide (hTERT) and Hsp70 peptide (HSP70) were obtained from Abcam (Britain). Sepharose 4B was obtained from GE Healthcare. All other reagents were purchased as analytical and molecular biology grades.Preparation and characterization of DEC205McAb coupled biomimetic Texosomes
The hTERT-HSP70 peptide complex was formed by incubating for 2 h at 37 °C in 1 mmol L−1 ADP and 1 mmol L−1 MgCl2 solution. hTERT-HSP70 loaded Texosomes were prepared using a layer-by-layer assembling method.15 Briefly, the inner lipid layer was prepared by W/O microemulsion. A surfactant (PC) and cosurfactant (Cremophor EL) were mixed in diethyl ether as an oil phase and the hTERT-HSP70 solution as the water phase was dropped into the oil phase until it changed from transparent to light blue opalescence. Pseudoternary phase diagrams were constructed to obtain the optimal ratio of oil and aqueous phase. The outer lipid layer composed of DOPE, DC-Chol and CHS with the weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was hydrated with a mixture of ethanol and deionized water (1
1 was hydrated with a mixture of ethanol and deionized water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) to form micelles at 60 °C for 30 min. Then, we mixed the inner and outer lipid liquid in a round-bottomed flask, and after vacuum evaporation of diethyl ether at 60 °C, Texosomes was finally obtained. DEC205 monoclonal antibodies were then covalently conjugated to the surface of the Texosomes by an activation step and a coupling step to obtain DEC205 monoclonal antibodies coupled biomimetic Texosomes (DEC205McAb Texosomes). Then the Texosomes and DEC205McAb Texosomes were lyophilized and stored at 4 °C for later use. The particle size was determined by a Dynamic Light Scattering Instrument (LS 320; Beckman, American) and the mean diameter was expressed as volume diameter. The zeta potential was measured by a zeta potential analyzer (Delsa 440SX; Beckman, American). The mean diameter of Texosomes and DEC205McAb Texosomes was 67.7 ± 8.22 nm and 88.3 ± 7.52 nm. They were cationic charged and the zeta potential was +32.2 ± 3.24 mV and +20.3 ± 2.67 mV. The encapsulation efficiency was 90.62 ± 4.35% and 92.56 ± 3.29% respectively. The conjugation efficiency of DEC205McAb Texosomes was 64.43 ± 6.11%.
2, v/v) to form micelles at 60 °C for 30 min. Then, we mixed the inner and outer lipid liquid in a round-bottomed flask, and after vacuum evaporation of diethyl ether at 60 °C, Texosomes was finally obtained. DEC205 monoclonal antibodies were then covalently conjugated to the surface of the Texosomes by an activation step and a coupling step to obtain DEC205 monoclonal antibodies coupled biomimetic Texosomes (DEC205McAb Texosomes). Then the Texosomes and DEC205McAb Texosomes were lyophilized and stored at 4 °C for later use. The particle size was determined by a Dynamic Light Scattering Instrument (LS 320; Beckman, American) and the mean diameter was expressed as volume diameter. The zeta potential was measured by a zeta potential analyzer (Delsa 440SX; Beckman, American). The mean diameter of Texosomes and DEC205McAb Texosomes was 67.7 ± 8.22 nm and 88.3 ± 7.52 nm. They were cationic charged and the zeta potential was +32.2 ± 3.24 mV and +20.3 ± 2.67 mV. The encapsulation efficiency was 90.62 ± 4.35% and 92.56 ± 3.29% respectively. The conjugation efficiency of DEC205McAb Texosomes was 64.43 ± 6.11%.
In vitro release
5 mL Aliquots of Texosomes and DEC205McAb Texosomes with the same amount of hTERT-HSP70 were transferred into the dialysis bags (molecular weight cut-off 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M wt) and dialyzed against 100 mL of phosphate buffer with a pH 7.4 at 37 ± 0.5 °C for 96 h under sink conditions. Periodically, 2 mL of aliquots were withdrawn at designated time points of 0.5, 2, 6, 8, 12, 24, 48, 72, 96 h and replaced with the equal volume of fresh medium, then filtered and assayed by Bradford method for drug release analysis. The cumulative hTERT-HSP70 release was plotted as a function of time.
000 M wt) and dialyzed against 100 mL of phosphate buffer with a pH 7.4 at 37 ± 0.5 °C for 96 h under sink conditions. Periodically, 2 mL of aliquots were withdrawn at designated time points of 0.5, 2, 6, 8, 12, 24, 48, 72, 96 h and replaced with the equal volume of fresh medium, then filtered and assayed by Bradford method for drug release analysis. The cumulative hTERT-HSP70 release was plotted as a function of time.
The cellular localization
NBD-PE as a fluorescence probe was added to prepare NBD labeled biomimetic Texosomes (NBD-Texosomes) and NBD labeled DEC205McAb coupled biomimetic Texosomes (NBD-DEC205McAb Texosomes) according to the above methods. Dendritic cells were seeded on a 6-well culture plate at a density of 2 × 105 cells per well for 24 h. After adding the NBD-Texosomes and NBD-DEC205McAb Texosomes with equivalent concentrations, the cells were incubated for 0.5 h, 1 h and 4 h at 37 °C. After the cells were soaked in 4% paraformaldehyde and treated with Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 258 at 37 °C for 30 min, the intracellular localization of NBD-Texosomes and NBD-DEC205McAb Texosomes was recorded using a confocal laser scanning microscopy (LSCM, Olympus, Japan).
258 at 37 °C for 30 min, the intracellular localization of NBD-Texosomes and NBD-DEC205McAb Texosomes was recorded using a confocal laser scanning microscopy (LSCM, Olympus, Japan).
DEC205McAb Texosome persistence at injection sites
Female BALB/c nude mice (18–22 g) were purchased from Beijing HFK Bioscience Co., Ltd. All mice were housed in the SPF II lab and given free access to food and water. All procedures were conducted in compliance with the Animal Ethical Guidelines for Investigations in Laboratory Animals and were approved by the Ethical Committee for Animal Experimentation of Shenyang Pharmaceutical University. The Texosomes and DEC205McAb Texosomes were labeled with DiR dye and injected into the abdominal subcutaneous tissue of nude mice in order to monitor the persistence at injection sites in vivo using an NIR fluorescence imaging system within 168 h after administration.Immunization studies
Female BALB/c mice (SPF, 18–22 g) were purchased from the animal center of Shenyang Pharmaceutical University. Animals were housed with free access to food and water. The tumor-bearing mice were produced by inoculating a suspension of H22 cells (3 × 107 cells per mL) subcutaneously into the right axillary fossa. When the tumor reached a volume of 50 mm3, the mice were respectively divided into six groups with six mice in each group. Mice were immunized 3 times at 0, 7 and 14 days by abdominal subcutaneous with different vaccine formulations, respectively saline, HSP70 solution, hTERT solution, hTERT-HSP70 solution, and Texosomes and DEC205McAb Texosome containing 100 μg kg−1 of antigen. Mice blood samples were then collected by retro-orbital puncture after 7, 14 and 21 days of primary immunization and sera was separated and stored at 70 °C for later analysis to detect the antibody level of IL-12 and IFN γ by ELISA. Tumor size and survival rate were measured every 2–3 days and calculated using the formula: volume = 0.5 × (width)2 × (length).Cell culture
The dendritic cells were purchased from JRDUN Biotechnology Co., Ltd. and cultured in RPMI 1640 Medium, supplemented with 10% fetal bovine serum (FBS), 100 U mL−1 penicillin, and 100 mg mL−1 streptomycin (PAA, Austria) in humidified air with 5% CO2 at 37 °C.Statistical analysis
All of the data are presented as the mean ± standard deviation (SD) and all experiments were performed with at least 3 independent repeats. Differences between groups were examined using Student’s t-test between 2 groups or one-way analysis of variance (ANOVA) among ≥3 groups.Results and discussion
In vitro release characteristics
The typical release profile of antigen–peptide complex from Texosomes and DEC205McAb Texosomes is shown in Fig. 1. The release of two groups was both very slow, and the cumulative release amounts from Texosomes and DEC205McAb Texosomes were only 20.87% ± 3.20% and 15.69% ± 2.09% at 96 h, respectively. The reasons for slow release were are described below. We prepared these carriers by a novel micro-emulsion and micelle layer-by-layer assembling method, and the antigen peptide complex had high encapsulation efficiency above 90%.15 That is to say that the tumor antigen was mostly retained in the inner aqueous phase of the bi-layer lipid membrane and rarely remained outside. It was difficult for the hTERT-HSP70 enclosed inside with a molecular weight of 71 kDa to leak through the membrane because of space hindrance, so the release rate was slow within 96 h all the way. Moreover, there was almost no effect of ligand modification on the release. Even if some of the hTERT-HSP70 remained outside, it was also difficult for this to be released due to electrostatic adsorption and the lipid membrane being electropositive while the antigen was negative, therefore the burst release effect did not exist. very suitable for uptake by dendritic cells, and enough was released and the antigens were efficient. Before DEC205McAb Texosomes was recognized, less the antigen was released at the injection site, and therefore more antigen was endocytosed by the immature dendritic cells. | ||
| Fig. 1 Cumulative release of hTERT-HSP70 from the Texosomes and DEC205McAb Texosomes in pH 7.4 PBS at 37 °C (mean ± SD, n = 6). | ||
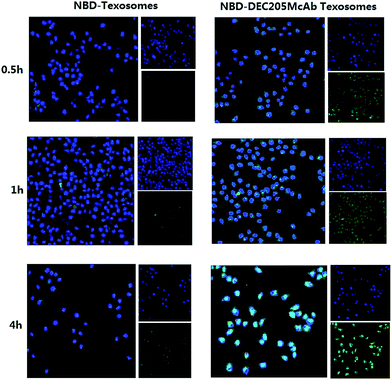
Uptake mechanism
To assess the cellular localization of NBD-Texosomes and NBD-DEC205McAb Texosomes in dendritic cells, we prepared cytospin samples and further analyzed their uptake mechanism by laser confocal microscopy (Fig. 2). Overall, the administration of NBD-Texosomes resulted in almost no green fluorescent signals and insignificant change of cellular uptake within 4 hours. The results demonstrated Texosomes could not be effectively internalized into dendritic cells. However, the green fluorescence intensity of the NBD-DEC205McAb Texosome group was enhanced from 0.5 h to 4 h and relatively strong fluorescence occurred at 4 h, which showed time dependent accumulation and broad distribution in dendritic cells during the incubation, that is to say that they were internalized via endocytosis with excellent cellular uptake. The significant difference between the NBD-Texosomes and NBD-DEC205McAb Texosomes in fluorescence intensity was obviously caused by the DEC205 monoclonal antibody modified on the carriers. DEC205 highly expressed receptors on the surface of DCs and DEC205 monoclonal antibody could combine with the receptors and greatly improve intracellular accumulation, therefore realized the aim of specific DCs targeting. All these above suggested that immature dendritic cells had more obviously phagocytic capacity on DEC205McAb Texosomes and they could be ingested by the way of receptor-mediated endocytosis and eventually entered the cell nucleus area.DEC205McAb Texosomes persistence at injection sites
To uncover the mechanisms underlying the enhanced immune responses elicited by the DEC205McAb Texosomes, we tracked and visualized the persistence of Texosomes and DEC205McAb Texosomes at the injection sites by in vivo imaging. As shown in Fig. 3, within 168 h the initial fluorescence region remained at the injection site in animals immunized with Texosomes, and there was no significant difference in fluorescence intensity. In contrast, the fluorescence region of DEC205McAb Texosomes gradually expanded and transferred to inguinal lymph nodes and then reduced at the injection sites for as long as 168 h (7 days) after injection, and so we designed the immune period for 7 days. Considering the significantly different persistence at the injection sites that existed between the Texosomes and DEC205McAb Texosomes, we concluded that these differences came from the level of the phagocytic ability of the dendritic cells.Texosomes would maintain their integrity and form a drug store just at injection site, as we have seen that the fluorescence intensity was still strong within 168 hours. Therefore we proposed that the prolonged retention time perhaps increase the uptaking chance by dendritic cells. But to the DEC205McAb Texosomes group, the mechanism of phagocytosis is different, because the modified ligand could specifically target the receptor on the surface of the dendritic cells and so promote the uptake efficiency by immature dendritic cells, and later these antigen presenting cells gradually matured and transferred to the lymph nodes. In the lymph nodes, the dendritic cells’ ability to uptake and process antigen was diminished, but the presentation ability was enhanced, so here the mature dendritic cells facilitated efficient antigen-loading to MHC molecules for antigen presentation to naïve T lymphocytes and eventually differentiated tumor specific cytotoxic T lymphocytes.16,17 Taking these results into consideration, it was suggested that DEC205 monoclonal antibody modified Texosomes not only provided effective uptake by dendritic cells but also completed the presentation of antigen information to T cells in lymph nodes.
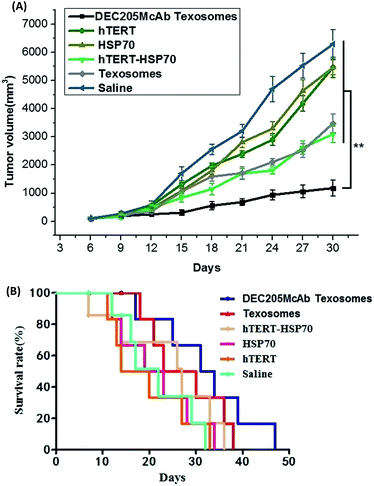
Anti-tumor immune responses in vaccinated mice
To evaluate the quality of the antibody response, the avidity between antigen and antibody in the serum was determined by ELISA. The anti-tumor effect of different formulations was further investigated in tumor-bearing mice after immunization with different vaccines. As we expected, saline, hTERT and HSP70 solution immunization failed to suppress tumor growth, and hTERT-HSP70 and Texosome immunization was moderately controlled for up to 12 days, whereas DEC205McAb Texosomes could obviously inhibit the tumor growth and induce tumor cell apoptosis, especially after 15 days (Fig. 4A). More importantly, the DEC205McAb Texosomes prolonged the survival period of tumor-bearing mice more effectively than hTERT-HSP70 and Texosomes did (Fig. 4B), indicating its potent anti-tumor effect. The saline, hTERT and HSP70 significantly dampened anti-tumor effect of the vaccination and reduced the overall survival. These data clearly demonstrate that the modification of DEC205 monoclonal antibodies on Texosomes significantly augmented the therapeutic efficacy of cancer vaccines.As is known, dendritic cells play a significant role in immunity because of their antigen presenting and lytic functions.18 Interleukin 12 (IL-12) is an interleukin that is naturally produced by dendritic cells after they were activated under antigenic stimulation.19 On one hand, IL-12 was involved in the differentiation of naive T cells into Th1 cells and promoted systemic immune response.20 On the other hand, IL-12 was known as a T cell-stimulating factor, which could accelerate the production of interferon-gamma (IFN-γ) as a Th1 signature cytokine and therefore evaluate the cellular immune response.21 At the same time, IFN-γ could also promote the generation and activation of antigen specific CTL and directly activates CTL cells, which are the effectors of cytotoxicity against tumors. In addition, we found that the anti-tumor effect of IL-12 was largely determined by the IFN γ and there also seemed to be a linked synergistic action between IL 12 and IFN γ on enhancing tumor-specific cytotoxic T lymphocyte responses.22,23 So in this study, we choose IL-12 and IFN γ levels to evaluate the immune effect. In the present study, the HSP70 group was barely able to stimulate the body to produce an immune response which would contribute to its poor immunogenicity (Fig. 5A and B). In contrast, hTERT, hTERT-HSP70 and Texosomes groups all could elicit moderate immune levels because hTERT is a universal tumor antigen and the bonding of HSP70 with hTERT could increase the immunogenicity of hTERT and promote the presenting of antigens. As for Texosomes, although Fig. 2 shows that non-targeted Texosomes exhibited poor cellular uptake compared to DEC205McAb Texosomes within 4 hours, from Fig. 3 we can see that Texosomes could stay at the injection site for at least 7 days and so increase the chance of phagocytosis by DCs, meanwhile the antigens could be protected in carriers effectively through encapsulation and so improve the antigen stability.24 As compared with the above groups, the strong production of IL12 as DCs activating signature cytokine and IFN γ as a tumor-specific CTL cytokine was significantly enhanced for DEC205McAb Texosomes group, consistent with our previous observation. These data demonstrated that the delivery of DEC205 monoclonal antibody coupled with biomimetic Texosomes synergistically evoked anti-tumor immune responses in vivo.
Based on the results in the present study, we therefore proposed the following model to explain the underlying mode of action by DEC205McAb Texosomes to promote tumor-specific immune responses (Fig. 6). Firstly, biomimetic DEC205McAb Texosomes loaded with hTERT-HSP70 could create an antigen depot at the injection site and provide a persistent immune stimulation to effectively activate dendritic cells. Then dendritic cells could proficiently uptake, process and highly express tumor-associated antigen peptide in the form of major histocompatibility complex class I molecules and meanwhile provide the co-stimulatory signals CD80/86 as well as an interleukin of interleukin 12.25,26 After combination with T cell receptors, tumor-specific cytotoxic T lymphocytes were activated, which consequently could specific recognize the tumor cells, launch an attack by injecting perforin and generate effective anti-tumor immune response.27,28
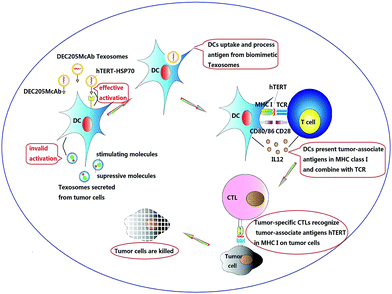 | ||
| Fig. 6 Schematic illustration of the proposed mode of action of DEC205 monoclonal antibody coupled biomimetic Texosomes. | ||
Conclusions
Currently, tumor induced dendritic cells dysfunction is recognized as the major obstacle for cancer vaccine-based immunotherapy. In the present study, we overcome the immune suppression by using DEC205McAb Texosomes loaded with tumor antigen hTERT-HSP70 as the carriers of a universal tumor vaccine. The effect of various antigen formulations on the anti-tumor immune responses was investigated. Our findings demonstrate that immature dendritic cells had more obviously phagocytic capacity on DEC205McAb Texosomes, and they could be ingested by the way of receptor-mediated endocytosis. Effective provision of both adequate initial antigen exposure and long-term antigen persistence induced DCs activation, antigen presentation as well as helper T cell differentiation in draining lymph nodes, therefore activate tumor-specific CTL reaction and finally lead to effective anti-tumor immune responses and tumor regression. Hence, researching the immune responses elicited by ligand-mediated biomimetic Texosomes might have significant implications for rational vaccine design and it is also expected to be a promising strategy to enhance the anti-tumor efficacy of therapeutic vaccines by modulating dendritic cells and overcoming tumor immuno-suppression.Acknowledgements
We are grateful to financial support from the Natural Science for Youth Foundation (No. 81302721, No. 81202483) and general project of Education Department of Liaoning Province (No. L2014383).References
- B. Pulendran and R. Ahmed, Nat. Immunol., 2011, 131, 509–517 CrossRef.
- V. Sokolova, T. Knuschke and A. Kovtun, et al., Biomaterials, 2010, 31, 5627–5633 CrossRef CAS PubMed.
- C. Bauer, M. Dauer and S. Saraj, Cancer Immunol. Immunother., 2011, 60, 1097–1107 CrossRef CAS PubMed.
- S. Baek, C. S. Kim and S. B. Kim, J. Transl. Med, 2011, 9, 178–184 CrossRef CAS PubMed.
- L. Evelyn, G. Victoria and E. Miriam, Mutat. Res., 2013, 752, 119–128 Search PubMed.
- A. Smogorzewska and T. Lange, Annu. Rev. Biochem., 2004, 73, 177–208 CrossRef CAS PubMed.
- M. C. Eugenia, M. T. Joseph and W. L. Meng, J. Mol. Biol., 2015, 427, 1575–1588 CrossRef PubMed.
- S. A. Lee, B. R. Kim and B. K. Kim, Biomaterials, 2013, 34, 7495–7505 CrossRef CAS PubMed.
- M. L. Temmerman, J. Rejman and J. Demeester, Drug Discovery Today, 2011, 16, 569–582 CrossRef PubMed.
- S. Kumar, A. C. Anselmo and A. Banerjee, et al., J. Controlled Release, 2015, 220, 141–148 CrossRef CAS PubMed.
- D. W. Greening, S. K. Gopal and R. Xu, Semin. Cell Dev. Biol., 2015, 40, 72–81 CrossRef CAS PubMed.
- T. Aaron, R. Jayakumar and M. S. Alexander, Adv. Drug Delivery Rev., 2013, 65, 357–367 CrossRef PubMed.
- B. Claudia and T. Thomas, Int. J. Biochem. Cell Biol., 2012, 44, 2060–2064 CrossRef PubMed.
- M. N. Leo, B. Jocelyn and J. D. Christopher, Vaccine, 2012, 30, 1624–1635 CrossRef PubMed.
- K. X. Li, S. S. Chang and Z. Y. Wang, Int. J. Pharm., 2015, 491, 105–112 CrossRef CAS PubMed.
- D. Yoonkyung, M. D. Arnaud and R. Seongho, Vaccine, 2012, 45, 6359–6367 Search PubMed.
- X. L. Zhang, J. Ma and M. Xu, Thromb. Res., 2015, 135, 352–361 CrossRef CAS PubMed.
- W. F. Zhang, L. Y. Wang and Y. Liu, Biomaterials, 2014, 35, 6086–6097 CrossRef CAS PubMed.
- S. W. Lee, H. J. Park and K. S. Lee, Biochem. Biophys. Res. Commun., 2015, 461, 86–94 CrossRef CAS PubMed.
- D. A. Blair, D. L. Turner and T. O. Bose, J. Immunol., 2011, 187, 2310–2321 CrossRef CAS PubMed.
- T. D. Fern andez, J. R. Pearson and M. P. Leal, Biomaterials, 2015, 43, 1–12 CrossRef CAS PubMed.
- A. B. Martine, A. Martino and C. B. Sven, J. Controlled Release, 2015, 216, 37–46 CrossRef PubMed.
- T. Yafang, P. G. Shou and Y. L. Benson, J. Allergy Clin. Immunol., 2012, 129, 1611–1620 CrossRef PubMed.
- S. Petra, F. Benjamin and J. Oliver, Radiother. Oncol., 2011, 101, 109–115 CrossRef PubMed.
- L. Thonur, D. M. Haig and J. Thomson, J. Comp. Pathol., 2012, 147, 296–304 CrossRef CAS PubMed.
- L. J. Cruz, R. A. Rosalia and J. W. Kleinovink, J. Controlled Release, 2014, 192, 209–218 CrossRef CAS PubMed.
- P. Nordly, E. M. Agger and P. Andersen, Pharm. Res., 2011, 28, 553–562 CrossRef CAS PubMed.
- T. Nakamura, R. Moriguchi and K. Kogure, Int. J. Pharm., 2013, 441, 476–481 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |