Preparation and characterization of bark-derived phenol formaldehyde foams
B. Lia,
S. H. Fenga,
H. S. Niasara,
Y. S. Zhanga,
Z. S. Yuana,
J. Schmidtb and
C. Xu*a
aInstitute for Chemicals and Fuels from Alternative Resources, Western University, London, Ontario, Canada N6A 5B9. E-mail: bli349@uwo.ca; cxu6@uwo.ca; Fax: +1-519-661-4016; Tel: +1-519-661-2111 ext. 86414
bFPInnovations 570, boul. Saint-Jean, Pointe-Claire, QC H9R 3J9, Canada
First published on 15th April 2016
Abstract
Bark-derived oils produced by hydrothermal liquefaction of outer and inner white birch bark in an ethanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) mixture were utilized for the synthesis of bio-based phenol formaldehyde (BPF) foamable resole resins with different levels of phenol substitution ratios (25 wt% and 50 wt%). Then BPF foams were successfully produced by mixing a blowing agent, a surfactant and a curing agent with the synthesized BPF resoles. The FT-IR analysis showed that BPF foamable resoles and BPF foams both have a very similar structure as a conventional phenol formaldehyde (PF) resole resin and foam. The obtained BPF foams displayed satisfactory compressive strength, elastic modulus, and thermal conductivity. The inner bark-derived oil was found to be more suitable than the outer bark-derived oil for the production of BPF foams, as the former resulted in foams with lower densities and tinier cell structure.
1, w/w) mixture were utilized for the synthesis of bio-based phenol formaldehyde (BPF) foamable resole resins with different levels of phenol substitution ratios (25 wt% and 50 wt%). Then BPF foams were successfully produced by mixing a blowing agent, a surfactant and a curing agent with the synthesized BPF resoles. The FT-IR analysis showed that BPF foamable resoles and BPF foams both have a very similar structure as a conventional phenol formaldehyde (PF) resole resin and foam. The obtained BPF foams displayed satisfactory compressive strength, elastic modulus, and thermal conductivity. The inner bark-derived oil was found to be more suitable than the outer bark-derived oil for the production of BPF foams, as the former resulted in foams with lower densities and tinier cell structure.
1. Introduction
Thermally insulating materials are important in diverse fields such as civil construction and manufacture of military aircrafts and marine vessels due to their ability to reduce energy consumption. Rigid closed cell phenolic foam has been attracting much attention as a thermal insulating material because of its low thermal conductivity and exceptional flame-retardant properties, including low flammability with no dripping during combustion, low smoke and toxicity (FST).1,2 For instance, phenolic foam composites used in building exterior walls and roofs could reduce the rate of heat transmission across these building elements and frequency of fire accidents.3 Moreover, the high chemical-resistance of phenolic foam and its low price make it attractive in applications where chemicals are presented.4The primary raw materials for phenolic foams – phenol and formaldehyde – are currently obtained from petroleum. Environmental and sustainability concerns the fluctuating cost of crude oil have renewed interest in using biomass as a source of oxygenated compounds such as phenols, organic acids and alcohols.5 Forest or agricultural lignocellulosic biomass typically contains 15–35% lignin.6 Lignin is an amorphous natural polymer composed of phenyl propane units and thus a potential source of phenolic compounds for the synthesis of bio-based phenolic resins.7 Bark is an abundant forestry residue in Canada, which is typically burned as a hog fuel for energy recovery.8 However, bark with a high lignin content (up to 40–50 wt%) is a potential feedstock for phenolic compounds after liquefaction into bio-oils.9
In the past decades, extensive studies had been performed on the synthesis of bio-based phenol formaldehyde (BPF) foams using bio-phenols. However, the phenol substitution in BPF foams is limited to about 30%, even though pretreatment was utilized, such as phenolation and oxidation, because of low reactivity and high molecular weight of BPF resole compared with commercial PF resin.10 Two approaches can be taken to the production of BPF foams with a higher phenol substitution ratio and more homogeneous cells and satisfactory properties. One approach is to boost the reactivity of the bio-based phenolic resin by improving the quality of bio-oils. The authors' group has been successful on direct liquefaction of bark or woody biomass in water/ethanol mixture to produce more reactive bio-oils for various applications9,11–14 including the synthesis of BPF resins.6,7,15–17 The obtained BPF resins have a phenol substitution ratio as high as 75% without sacrificing the physical/chemical properties of the resins when compared with conventional PF resin. The other approach is to manipulate the parameters of foaming process in accordance to the characteristics of BPF resole resins, in order to develop an optimal foaming technology for BPF foams. Phenolic foam is commonly produced by dispersing a gas through a liquid foamable phenolic resin phase followed by stabilizing the foaming system. The foam formation process involves bubble formation, bubble growth, and bubble stabilization.1 Delaying the bubble formation and growth during foaming process could provide more time for the crosslinking of the low reactivity BPF resin. In this case, the use of the blowing agent with a high boiling point is preferable for slowing-down cell formation during the crosslinking of BPF foamable resoles under acidic condition. Besides, it is desirable to add more acid to enhance the crosslinking of BPF resin and avoid excess coalescence of bubbles, thereby controlling the process of bubble stability for the production of BPF foam with homogeneous and fine cell structure.
Based on our preliminary studies, white birch bark was chosen as feedstock to produce a high quality phenolic bio-oil via hydrothermal liquefaction in this study. BPF foamable resoles were prepared through the resinification of bark-derived oil, phenol, and formaldehyde under alkaline condition. BPF foams were then produced by mixing a blowing agent, a surfactant and a curing agent with the synthesized BPF foamable resoles. The obtained resoles and foams were characterized in terms of physical, mechanical, thermal and morphological properties.
2. Experimental
2.1 Materials
White birch bark used in this study was obtained from a local sawmill in Northwestern Ontario, Canada. The white birch bark was separated to inner bark and outer bark and air dried, then ground into particles of less than 20 mesh, and finally oven dried at 105 °C for 24 h before use. The white birch barks consists of 37.98% cellulose, 28% hemicellulose, and 34.02% lignin after extraction.9 The extractive content of the bark (the ground air dried inner and outer white birch barks) was determined through lixiviation in acetone, ethanol, and ethanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v), respectively, at room temperature for 24 hours. The extractive mass was obtained by rotary evaporation of extractive filtrate to completely remove the solvent at 50–70 °C under reduced pressure, and the extractive content (%) was calculated based on the mass of oven-dried bark, as shown later in Table 1. ACS reagent-grade ethanol (Fisher Scientific, Batesville, IN), acetone (Fisher Scientific, Fair Lawn, NJ), solid phenol crystal (99%, J. T. Baker, Phillipsburg, NJ), sodium hydroxide solution (ca. 50%, Ricca Chemical Co., Arlington, TX), formaldehyde (ca. 37%, Anachemia, Montreal, QC), and Acetic acid (ca. 99.7% Caledon Laboratory Chemicals, Sigma-Aldrich). Surfactant: polyetherpolysiloxane-copolymer, Tegostab B 8462; blowing agent: hexane (ca. 98.5%, VWR International LLC); curing catalyst/agent: p-toluenesulfonic acid (ca. 98%, Sigma-Aldrich) and glycerol (ca. 99.5%, Caledon Laboratory Chemicals).
2, v/v), respectively, at room temperature for 24 hours. The extractive mass was obtained by rotary evaporation of extractive filtrate to completely remove the solvent at 50–70 °C under reduced pressure, and the extractive content (%) was calculated based on the mass of oven-dried bark, as shown later in Table 1. ACS reagent-grade ethanol (Fisher Scientific, Batesville, IN), acetone (Fisher Scientific, Fair Lawn, NJ), solid phenol crystal (99%, J. T. Baker, Phillipsburg, NJ), sodium hydroxide solution (ca. 50%, Ricca Chemical Co., Arlington, TX), formaldehyde (ca. 37%, Anachemia, Montreal, QC), and Acetic acid (ca. 99.7% Caledon Laboratory Chemicals, Sigma-Aldrich). Surfactant: polyetherpolysiloxane-copolymer, Tegostab B 8462; blowing agent: hexane (ca. 98.5%, VWR International LLC); curing catalyst/agent: p-toluenesulfonic acid (ca. 98%, Sigma-Aldrich) and glycerol (ca. 99.5%, Caledon Laboratory Chemicals).
2.2 Preparation of bark-derived oils by hydrothermal liquefaction
Hydrothermal liquefaction of white birch barks (inner bark and outer bark) was performed in a 500 mL stirred reactor. In a typical run, 25 g bark and 250 mL ethanol–water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) mixed solvent were charged into the reactor, followed by three cycles of evacuation-N2 purge for removing the residual air in the reactor. Then the reactor was pressurized to 2.0 MPa using N2. The reactor was heated to 300 °C under constant stirring and further kept for 15 min at the reaction temperature. The reaction conditions used in this study were adopted from our previous study on bark liquefaction.9 Then liquefaction reaction was stopped by quenching the reactor in a water/ice bath. The liquid and solid residues in the reactor were washed using acetone, and collected and filtered. The bark-derived oil was obtained through evaporation of filtrate and stored in the fridge for the preparation of BPF foamable resole resins.
50, v/v) mixed solvent were charged into the reactor, followed by three cycles of evacuation-N2 purge for removing the residual air in the reactor. Then the reactor was pressurized to 2.0 MPa using N2. The reactor was heated to 300 °C under constant stirring and further kept for 15 min at the reaction temperature. The reaction conditions used in this study were adopted from our previous study on bark liquefaction.9 Then liquefaction reaction was stopped by quenching the reactor in a water/ice bath. The liquid and solid residues in the reactor were washed using acetone, and collected and filtered. The bark-derived oil was obtained through evaporation of filtrate and stored in the fridge for the preparation of BPF foamable resole resins.
2.3 Synthesis of BPF foamable resole resins
The conventional PF resole resin with the P/F ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 prepared as the reference sample. For the bio-based resoles, however, we did not using the same high F/P ratio mainly due to the fact that the bark-oil (lignin-based oil) has less reactive para- and ortho-positions on its benzene ring when compared with pure phenol. The molecular weight (Mw) of phenol is 94 g mol−1 with 3 reactive positions on the benzene ring, while we assume that Mw of monomer lignin unit (i.e., coniferyl alcohol unit, C10H14O3) is approximately 184 g mol−1 with 1 reactive position on the ortho-position of the benzene ring. Thus, apparently the bark lignin-derived oil has less reactive cites compared with pure phenol when reacting with formaldehyde. In order to control the free formaldehyde content of the BPF resoles to an acceptably lower level, we purposely lowered the F/P molar ratio to be 1.3
1.8 prepared as the reference sample. For the bio-based resoles, however, we did not using the same high F/P ratio mainly due to the fact that the bark-oil (lignin-based oil) has less reactive para- and ortho-positions on its benzene ring when compared with pure phenol. The molecular weight (Mw) of phenol is 94 g mol−1 with 3 reactive positions on the benzene ring, while we assume that Mw of monomer lignin unit (i.e., coniferyl alcohol unit, C10H14O3) is approximately 184 g mol−1 with 1 reactive position on the ortho-position of the benzene ring. Thus, apparently the bark lignin-derived oil has less reactive cites compared with pure phenol when reacting with formaldehyde. In order to control the free formaldehyde content of the BPF resoles to an acceptably lower level, we purposely lowered the F/P molar ratio to be 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to some previous work by the authors' group and some literature work.
1 according to some previous work by the authors' group and some literature work.
The synthesis of BPF foamable resoles with different phenol substitution levels using the bark derived bio-oils was carried out in a 1000 mL three-neck flask equipped with a thermometer, addition funnel, cooling condenser, and water bath with a magnetic stirrer. The ratio of (phenol + lignin) to formaldehyde in the BPF resoles synthesis was fixed at 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In a typical run for BPF resin synthesis, 120 g bio-oil and phenol, 30 g water, 10.2 g 50 wt% sodium hydroxide solution were charged into a flask and then the flask was heated to 80 °C for 1 hour with magnetic stirring. Next, 135 g formaldehyde (ca. 37%) was added dropwise to the flask. At the same time, the temperature of the water bath was adjusted to 86 °C. After reaction for 2 h, the obtained resole was cooled down in a water bath until reaching 60 °C. The pH of resole was then adjusted to 5.5–6.5 by adding acetic acid. Finally, the resole was concentrated by evaporation under reduced pressure until the solid content of resin reached 70–85 wt%.
1. In a typical run for BPF resin synthesis, 120 g bio-oil and phenol, 30 g water, 10.2 g 50 wt% sodium hydroxide solution were charged into a flask and then the flask was heated to 80 °C for 1 hour with magnetic stirring. Next, 135 g formaldehyde (ca. 37%) was added dropwise to the flask. At the same time, the temperature of the water bath was adjusted to 86 °C. After reaction for 2 h, the obtained resole was cooled down in a water bath until reaching 60 °C. The pH of resole was then adjusted to 5.5–6.5 by adding acetic acid. Finally, the resole was concentrated by evaporation under reduced pressure until the solid content of resin reached 70–85 wt%.
2.4 Preparation of BPF foams
The formula for PF foam or BPF foam involves two fractions. Fraction-1 is a mixture of a surfactant, a blowing agent and a foamable resole resin. For instance, to prepare fraction-1 for the conventional PF foam, 1.2 g polyetherpolysiloxane-copolymer and 4 g hexanes (50–70 °C) as a blowing agent was added into 40 g PF foamable resole in a paper cup, stirred with a mechanical stirrer. To prepare fraction-2, 5.6 g p-toluenesulfonic acid, 2.4 g distilled water and 2 g glycerol were mixed. Subsequently, fraction-2 was blended with fraction-1 by vigorous agitation for 30 seconds at room temperature before it was moved into a preheated oven at 60–80 °C for 30 min. The BPF foams with various phenol substitution levels were prepared in the same formula except that different amounts of p-toluenesulfonic acid (6.2 g for BPF foams with 25% phenol substitution and 6.8 g for BPF foams with 50% phenol substitution) were used due to the much lower reactivity of BPF resole resins compared with the PF resin.2.5 Characterizations of BPF and PF foamable resole resins
The pH values of all the prepared foamable resole resins were measured with a pH meter (SympHony, H10P, VWR). The viscosity of all the BPF resoles was measured at 60 °C on a Brookfield CAP 2000+ viscometer (Brookfield Engineering Laboratories, Middleboro, MA). Solid contents (nonvolatile contents) of all resins were determined at 125 °C for 105 min according to ASTM D 4426-01. The molecular weights and their distributions of the prepared foamable resoles were measured on a Waters Breeze gel permeation chromatograph (GPC) (1525 binary HPLC pump; UV detector at 270 nm; Waters Styrange HR1 column at 40 °C) using THF as the eluant at a flow rate of 1 mL min−1. The GPC was calibrated using polystyrene standards. Free formaldehyde level in the foamable resoles was analyzed by the method in accordance to ISO 9397 (hydroxylamine hydrochloride method). Free phenol content of the resoles was measured with a Gas Chromatography/Mass Spectrometry (GC/MS) (Agilent 7890B GC, 5977AMSD) equipped with an Agilent DB-35ms column, pre-calibrated with pure phenol compound. For the GC/MS analysis, 0.04 g resin sample was diluted with 1.00 g ethanol to prepare a diluted sample for injection. The temperature program for the GC was: 120 °C (hold for 2 min) → 250 °C (50 °C min−1, hold for 2 min).2.6 Characterizations of BPF and PF foams
Apparent density of the foams was determined in accordance with ASTM D1622. The samples were cut in cubic specimens. A minimum of three specimens with the same formula were tested. FTIR spectra were collected on a Perkin Elmer Frontier FTIR to characterize the bio-phenols, PF/BPF resoles and, the prepared PF/BPF foams, using transmission mode in the wavenumber range of 550 to 4000 cm−1. Thermal conductivity of the foams was measured with a KD2 Pro thermal properties analyzer at room temperature using needle probes. Three specimens were tested for each foam sample. Compressive strength of the foams was measured on an ADMET Expert 7600 universal test machine according to ASTM D1621, with a fixed strain rate at 2.5 mm min−1. Compressive strengths of the foams were determined at various strain and compressive modulus were calculated from the slope of the stress–strain curves, and a minimum of three specimens were tested for each type of foam. Morphology of foams was observed by Zeiss 1540XB FIB-SEM.3. Results and discussion
3.1 Mechanism for BPF foam production
BPF foam production process in this work involves three stages, bark liquefaction for bio-oils, BPF foamable resole resin synthesis, and BPF resole foaming process. The first process is the hydrothermal liquefaction of bark. The bark mainly consists of cellulose, hemicellulose, and lignin, which are all macromolecules and can be degraded into oligomeric and monomeric compounds through thermochemical technologies. For instance, Fig. 1 shows the possible cleavage of β-O-4 linkage in bark lignin.14Then, the bark-derived PF resole resins can be synthesized by reacting formaldehyde at the ortho-position of phenolic compounds in the bark-derived oils, which is quite similar to the reaction between phenol and formaldehyde. Fig. 2 illustrates a possible reaction for the synthesis of BPF foamable resole resins using bark-derived oil under alkaline conditions.18–20
Furthermore, the BPF foams are produced by mixing the foaming agent, surfactant, and curing agent. Fig. 3 shows the possible crosslinking reaction in the BPF foam production using the BPF foamable resoles.18 Similar to the conventional PF resole, the BPF foamable resoles are mainly cross-linked through methylene and ether linkages between benzene rings during curing, which can be proved by the similar FT-IR spectra of the synthesized BPF foams and the reference PF foam (see Fig. 2 and 3).
3.2 Characterization of bark-derived BPF foamable resoles
The extractive content of feedstock was given in the Table 1, depending on the extraction solvents. As shown in the Table, extractives yield from the soxhlet extraction of outer birth bark using acetone, ethanol, and ethanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) solvents were 22.12%, 26.38%, and 26.62%, respectively, which are much higher than that from the inner bark (2.81%, 3.72%, and 6.16%), correspondingly. In addition, the chemical compositions of outer white birch bark and inner white birch bark vary a lot. Outer bark is composed of more cellulose while the inner bark contains more lignin.21 Thus, inner bark and outer bark were separately used for bio-oil production in this study.
2, v/v) solvents were 22.12%, 26.38%, and 26.62%, respectively, which are much higher than that from the inner bark (2.81%, 3.72%, and 6.16%), correspondingly. In addition, the chemical compositions of outer white birch bark and inner white birch bark vary a lot. Outer bark is composed of more cellulose while the inner bark contains more lignin.21 Thus, inner bark and outer bark were separately used for bio-oil production in this study.
The obtained bio-crude oils were used as bio-phenols to substitute phenol at a phenol substitution level of 25 wt% and 50 wt%, respectively, for the preparation of foamable BPF resoles. Although conventional PF resoles are commonly synthesized at a formaldehyde/phenol (F/P) molar ratio of 1.6–2.1,22 this study applied F/P molar ratio of 1.3 for all the BF and BPF resole synthesis, due to the less reactive sites in the bio-phenols (para- and ortho-positions) compared with petroleum based phenol. The resulted bark-derived BPF resoles are denoted as: 25% BPF-outer bark, 50% BPF-outer bark, 25% BPF-inner bark and 50% BPF-inner bark. Physical properties of PF and bark-derived BPF foamable resoles, e.g., pH value, viscosity, solid content, free formaldehyde, and free phenol contents, are presented in Table 2.
| Resoles | pH valuea | Viscositya (P, at 60 °C) | Solid contenta (wt%) | Free formaldehyde contenta (wt%) | Free phenol contenta (wt%) |
|---|---|---|---|---|---|
| a Each value represents an average of three samples.b 25% BPF-outer bark means BPF resole with 25% phenol substitution using outer bark-derived oil.c 50% BPF-outer bark means BPF resole with 50% phenol substitution using outer bark-derived oil.d 25% BPF-inner bark means BPF resole with 25% phenol substitution using inner bark-derived oil.e 50% BPF-inner bark means BPF resole with 50% phenol substitution using inner bark-derived oil. | |||||
| PF | 5.67 ± 0.02 | 1.35 ± 0.05 | 78.3 ± 0.9 | 0.68 ± 0.02 | 3.10 ± 0.01 |
| 25% BPF-outer barkb | 5.76 ± 0.01 | 1.69 ± 0.06 | 73.7 ± 0.8 | 0.31 ± 0.03 | 2.12 ± 0.01 |
| 50% BPF-outer barkc | 5.56 ± 0.01 | 1.83 ± 0.08 | 77.5 ± 0.6 | 1.12 ± 0.04 | 0.92 ± 0.01 |
| 25% BPF-inner barkd | 5.85 ± 0.02 | 1.59 ± 0.04 | 76.9 ± 0.9 | 0.12 ± 0.03 | 1.71 ± 0.02 |
| 50% BPF-inner barke | 5.54 ± 0.01 | 1.78 ± 0.06 | 74.4 ± 0.4 | 0.98 ± 0.05 | 0.44 ± 0.03 |
The pH values of all resoles are very similar, within the narrow range of 5.5–5.9. Since after the resole synthesis, the pH values of all resoles were adjusted to 5.5–6.5 by adding acetic acid. The solid contents of all resoles are also in a narrow range (73.67–79.34 wt%) controlled through the rotary evaporation to remove some water. As confirmed in some previous work, solid content of 70–80 wt% was considered to be most preferable for BPF foams production, because the viscosity of the resole could increase rapidly if the solid content of BPF resoles is over 80%, bringing about a great handling difficulty during the mixing process.23 However, if the solid content of the resole is too low, the presence of excess water could cause perforations in the cell walls and even contribute to the rupturing of cell walls.23 It should be noted that the solid content (74.4 ± 0.4 wt%) of the BPF resole with 50% phenol substitution using inner bark-derived oil was purposely controlled to be slightly lower than that (76.9 ± 0.9 wt%) of the BPF resole with 25% phenol substitution using inner bark-derived oil, in order to lower its viscosity, so as to facilitate the foaming process for BPF resins with a high phenol substitution ratio. Free formaldehyde contents of the resoles range from 0.09 wt% (PF resole) to 0.12 wt% (50% BPF-outer bark). Generally, the free formaldehyde content the resole increased with the increasing phenol substitution level, suggesting lower reactivity of bark-derived bio-oil than phenol. Free phenol content in BPF resoles decreased with increasing the phenol substitution level as expected, because phenol is more reactive towards formaldehyde than the bio-phenols (bark-derived bio-oil), as similarly observed in a previous study.6
Molecular weights and distributions of all resole resins in comparison with the bark-derived bio-oils are shown in Table 3. The inner bark-derived bio-oil has a slightly smaller average molecular weight than the outer bark-derived bio-oil. Compared with the original bio-oils, all the BPF resoles have a much higher weight average molecular weight (Mw) or number average molecular weight (Mn), implying successful resinification reactions between the bio-oil and formaldehyde. It is also obvious that, the values of Mn, Mw and polydispersity for the BPF resole resins are higher those of the PF resole. The values increase with increasing the phenol substitution level, which can be explained by the large molecular weights of the bark-derived oils and broader molecular weight distribution.6
| Samples | Mn (g mol−1) | Mw (g mol−1) | PDI |
|---|---|---|---|
| Inner bark bio-oil | 292 | 841 | 2.88 |
| Outer bark bio-oil | 311 | 1076 | 3.45 |
| PF resole | 310 | 1220 | 3.94 |
| 25% BPF-inner bark | 603 | 1625 | 2.69 |
| 50% BPF-inner bark | 843 | 2197 | 2.61 |
| 25% BPF-outer bark | 624 | 1742 | 2.79 |
| 50% BPF-outer bark | 790 | 2377 | 3.01 |
Fig. 4 illustrates FTIR spectra of the bark-derived BPF resoles and the conventional PF resole. Clearly the FTIR spectra of all BPF resoles are similar to that of the conventional PF resole, suggesting that the BPF resoles have similar molecular structure as the conventional PF resole. All resoles have typical adsorption peaks of hydroxyl group at 3100–3700 cm−1 (stretching), C–H stretching at 2800–2950 cm−1, C–O stretching at 1025 cm−1, and aromatic C–H at 828/754/694 cm−1. Nevertheless, the FTIR spectra of the BPF resoles exhibit some differences from that of the conventional PF resole due to the presence of complicated linkages in the bio-oils component. For example, the intensities of characteristic peaks of bark-derived BPF resoles at 1600, 1508, 1450, 1223, 828, 754, and 694 cm−1 are weaker at a higher phenol substitution level, because of less phenolic/aromatic structure in bark-derived oils compared with petroleum-based phenol, as similarly reported in some previous studies.6,7
3.3 PF and BPF foams characterization
The FTIR spectra of the bark-derived BPF foams and the conventional PF foam are compared in Fig. 5. Again, the spectra of all BPF foams are very similar to that of the conventional PF foam, implying that the BPF foams have a similar chemical structure as the conventional PF foam. All of the foams have the typical hydroxyl group absorption between 3300 and 3400 cm−1, C–H stretching between 2820 and 2926 cm−1, aromatic rings between 1405 and 1510 cm−1, phenolic C–O stretching at 1204 cm−1, C–O stretching between 1000 and 1050 cm−1, aromatic C–H between 660 and 845 cm−1. It is also obvious that the spectra of the foams are similar to those of the resoles, if comparing Fig. 6 and 5.Table 4 shows the densities, compressive strengths, elastic moduli, and thermal conductivities of the bark-derived BPF foams compared with the conventional PF foam. The densities of inner-bark BPF foams are generally smaller than those of outer-bark BPF foams, which is probably due to the fact that the outer bark of white birch contains 4–7 times as many extractives as the inner bark (Table 1). The extractives would affect resin synthesis and foam formation, and increase the foam density.24 As a result, liquefied inner bark of birch is a more suitable source of bio-phenols for the production of low-density BPF foams. On the other hand, as clearly shown in Table 4, the density of the BPF foams is much higher compared to that of the conventional PF, and the densities of BPF foams increase with increasing the phenol substitution ratio. This finding could be resulted from the poorer foamability of the BPF resole due to its higher viscosity (Table 2), larger Mw (Table 3).
| Foam sample | Densitya (kg m−3) | Compressive strength at 10% straina (MPa) | Compressive strength at 20% straina (MPa) | MOE elasticitya (MPa) | Thermal conductivitya (W m−1 k−1) |
|---|---|---|---|---|---|
| a Each value represents an average of five samples. | |||||
| PF | 43.4 ± 1.3 | 0.19 ± 0.02 | 0.20 ± 0.02 | 3.13 ± 0.10 | 0.034 ± 0.001 |
| 25% BPF-inner bark | 78.2 ± 2.3 | 0.44 ± 0.04 | 0.55 ± 0.03 | 7.63 ± 0.91 | 0.038 ± 0.002 |
| 50% BPF-inner bark | 134.4 ± 4.1 | 0.79 ± 0.06 | 0.89 ± 0.05 | 10.19 ± 1.41 | 0.043 ± 0.005 |
| 25% BPF-outer bark | 89.3 ± 3.0 | 0.55 ± 0.04 | 0.63 ± 0.02 | 9.31 ± 1.10 | 0.040 ± 0.003 |
| 50% BPF-outer bark | 174.1 ± 5.5 | 1.00 ± 0.11 | 1.19 ± 0.07 | 14.61 ± 1.68 | 0.051 ± 0.004 |
The compressive strengths at 10% and 20% strain and modulus of elasticity of the foams increase dramatically with increasing the phenol substitution level, which could be mainly due to the fact that the BPF foam with a higher phenol substitution level has a higher density and thus better compressive properties. The other possible reason could be related to the branched structure of aromatics and alkyl side chains in the bio-oil that contributes to the larger strength of the BPF foams.25 It is interesting observation that the compressive strengths of the foams prepared from outer bark are better than those prepared form inner bark. This is likely because mechanical strength properties are generally proportional to the foam density,18 and as shown in Table 4 the BPF foams-outer bark have a higher foam density than the BPF foams-inner bark, which might account for their higher compressive strength.
Thermal conductivities of the BPF foams and the conventional PF foams are compared in Table 3. Thermal conductivity of all foams ranges from 0.03 W m−1 k−1 to 0.05 W m−1 k−1, but the value increases with increasing foam density or phenol substitution level in the BPF resole. Nevertheless, the thermal conductivities of all bark-derived BPF foams prepared in this study are comparable to those of most phenolic foams (0.04–0.08 W m−1 k−1 for densities 40–200 kg m−3).26
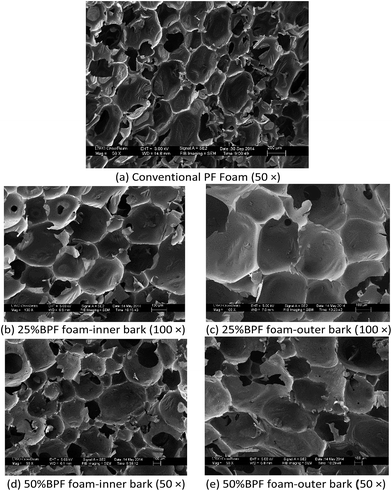
The SEM morphology of the BPF foams and conventional PF foam is shown in Fig. 6. It can be observed that all BPF and PF foams display satisfactory cell structure and mostly closed cells, although some perforations and ruptures can be found in these foams, which is due to the presence of water27 and the cutting operation for sample preparation. The BPF foams show better and smoother cell structure than the reference PF foam, which is probably due to the fact that BPF resins have higher viscosity than PF resin to entrap the bubble. Thus, that would facilitate stabilization of the foam before curing. The BPF foams-inner bark (Fig. 6b and d) show finer and more uniform cell structure than the BPF foams-outer bark (Fig. 6c and e), suggesting that the inner bark oil is more reactive than the outer bark oil. Accordingly, the BPF foams-outer bark would take more time in the resin curing process, which makes it difficult to entrap the blowing agent and keep the cell walls from cell coalescence. The 25% BPF foams (Fig. 6b and c) exhibit more uniform pore structure than the 50% BPF foams (Fig. 6d and e), most likely because the bark-derived oil has less reactive para- and ortho-position in the benzene ring compared with petroleum-based phenol.
4. Conclusions
Bark-derived oils obtained through hydrothermal liquefaction of inner/outer white birch barks were successfully applied in synthesis of BPF foamable resoles and foams at a phenol substitution up to 50 wt% employing a new foaming technology. The following conclusions can be drawn from this study:(1) Mw, Mn and PDI of the birch inner bark-derived oil are lower than those of the birch outer bark-derived oil. Correspondingly, Mw, Mn and PDI of the BPF resoles with the inner bark-derived oil are lower than those of the BPF resoles with the outer bark-derived oil;
(2) BPF resoles and BPF foams have very similar chemical structure as that of the conventional PF resole/foam;
(3) The prepared BPF foams have satisfactory compressive strength, modulus of elasticity, thermal conductivity, and cell structure. The 50% BPF foams show higher compressive strength and elastic modulus than the conventional PF foam or 25% BPF foams, while the 25% BPF foams demonstrate better uniformity of cell structure and lower density than 50% BPF foams. The BPF foams using bio-oil derived from inner bark have lower thermal conductivity and more uniform cell structure than the BPF foams using bio-oil from outer bark.
(4) Although bio-oils derived from both inner and outer bark of birch can be utilized as bio-phenols for the synthesis of BPF resoles/foams, the inner birch bark-derived bio-oil proved to be more suitable than the outer birch bark-derived bio-oil for the production of low-density BPF foams with finer cell structure.
Acknowledgements
The financial support for this work was mainly from NSERC Biomaterials and Chemicals Strategic Research Network (Lignoworks) partnered with FPInnovations, Lignol and Weyerhaeuser. One of the authors (C. X.) also gratefully acknowledges the funding from NSERC and Ontario government via an NSERC Discovery Grant, the NSERC/FPInnovations Industrial Research Chair program and an ORF-RE grant in Forest Biorefinery.Notes and references
- D. Klempner, V. Sendijarevic and R. M. Aseeva, Handbook of polymeric foams and foam technology, Hanser Publishers, Munich Cincinnati, 2nd edn, 2004 Search PubMed
.
- L. Pilato, Phenolic resins: a century of progress, Springer, 2010 Search PubMed
.
- D. L. Jayamaha, Energy-efficient building systems, Mcgraw-hill publishing Company, 2007 Search PubMed
.
- H. Shen, Ph.D dissertation, University of Southern California, 2003
.
- C. Amen-Chen, H. Pakdel and C. Roy, Bioresour. Technol., 2001, 79, 277–299 CrossRef CAS PubMed
.
- S. Cheng, I. D'Cruz, Z. Yuan, M. Wang, M. Anderson, M. Leitch and C. C. Xu, J. Appl. Polym. Sci., 2011, 121, 2743–2751 CrossRef CAS
.
- M. Wang, C. C. Xu and M. Leitch, Eur. Polym. J., 2009, 45, 3380–3388 CrossRef CAS
.
- C. Xing, J. Deng, S. Zhang, B. Riedl and A. Cloutier, For. Prod. J., 2006, 56, 64–69 Search PubMed
.
- S. Feng, Z. Yuan, M. Leitch and C. C. Xu, Fuel, 2014, 116, 214–220 CrossRef CAS
.
- L. Hu, Y. Zhou, R. Liu, M. Zhang and X. Yang, Ind. Crops Prod., 2013, 44, 364–366 CrossRef CAS
.
- C. Xu and T. Etcheverry, Fuel, 2008, 87, 335–345 CrossRef CAS
.
- C. Xu and N. Lad, Energy Fuels, 2008, 22, 635–642 CrossRef CAS
.
- C. Xu and J. Lancaster, Water Res., 2008, 42, 1571–1582 CrossRef CAS PubMed
.
- S. Cheng, I. D'cruz, M. Wang, M. Leitch and C. Xu, Energy Fuels, 2010, 24, 4659–4667 CrossRef CAS
.
- M. Wang, C. C. Xu and M. Leitch, J. Ind. Eng. Chem., 2009, 15, 870–875 CrossRef CAS
.
- M. Wang, C. C. Xu and M. Leitch, Bioresour. Technol., 2009, 100, 2305–2307 CrossRef CAS PubMed
.
- M. Wang, Z. Yuan, S. Cheng, M. Leitch and C. C. Xu, J. Appl. Polym. Sci., 2010, 1191–1197 CAS
.
- M. N. Mohamad Ibrahim, N. Zakaria, C. S. Sipaut, O. Sulaiman and R. Hashim, Carbohydr. Polym., 2011, 86, 112–119 CrossRef
.
- C. A. Fyfe, M. S. McKinnon, A. Rudin and W. J. Tchir, Macromolecules, 1983, 16, 1216–1219 CrossRef CAS
.
- S. Laurichesse and L. Avérous, Prog. Polym. Sci., 2014, 39, 1266–1290 CrossRef CAS
.
- S. Feng, S. Cheng, Z. Yuan, M. Leitch and C. C. Xu, Renewable Sustainable Energy Rev., 2013, 26, 560–578 CrossRef CAS
.
- V. Coppock, R. Zeggelaar, R. Takahashi and T. Kato, European Pat., 1922356B1, 2007
.
- E. W. Kifer, J. P. Colton and J. T. Stickel, US Pat., 4956394, 1990
.
- M. M. O'Connell, M. D. Bentley, C. S. Campbell and B. J. Cole, Phytochemistry, 1988, 27, 2175–2176 CrossRef
.
- L. Hu, Y. Zhou, M. Zhang and R. Liu, BioResources, 2012, 7, 554–564 CAS
.
- G. Tondi, W. Zhao, A. Pizzi, G. Du, V. Fierro and A. Celzard, Bioresour. Technol., 2009, 100, 5162–5169 CrossRef CAS PubMed
.
- J. D. Carlson, E. W. Kifer, V. J. Wojtyna and J. P. Colton, US Pat., 4539338, 1985
.
| This journal is © The Royal Society of Chemistry 2016 |