Improved performances of inkjet-printed poly(3-hexylthiophene) organic thin-film transistors by inserting an ionic self-assembled monolayer
Abstract
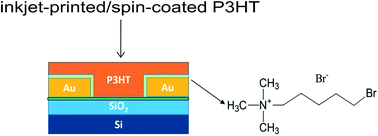
Inkjet printing is a promising technology because of the material conservation and facile patterning compared with other solution-processed techniques, facilitating the scalable fabrication and commercialization of organic electronics. In this study, organic thin-film transistors (OTFTs) based on poly(3-hexylthiophene) (P3HT) by inkjet printing were fabricated and explored by electrical analysis and morphological characterization. By optimizing the processing conditions, the comprehensive performance in terms of the field-effect mobility of inkjet-printed P3HT-based OTFTs was comparable to those of spin-coated P3HT-based OTFTs. More importantly, with the employment of an electrode buffer layer, namely Br(CH2)5N(CH3)3Br, the field-effect mobilities of both spin-coated and inkjet-printed OTFTs were improved in accordance with the expectations, resulting from the reduced contact resistance and improved film quality.

 Please wait while we load your content...
Please wait while we load your content...