Esterase-responsive polymeric prodrug-based tumor targeting nanoparticles for improved anti-tumor performance against colon cancer†
Gang Pan‡
a,
Yi-jie Bao‡a,
Jie Xu‡a,
Tao Liub,
Cheng Liub,
Yan-yan Qiub,
Xiao-jing Shia,
Hui Yua,
Ting-ting Jiac,
Xia Yuanc,
Ze-ting Yuanb,
Pei-hao Yin*ad and
Yi-jun Cao*a
aDepartment of General Surgery, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 200062, China. E-mail: caoyjxw@126.com; Fax: +86-21-52665957; Tel: +86-21-22233222-21201
bCentralab, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 200062, China
cDepartment of Pharmacy, Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 200062, China
dInterventional Cancer Institute of Chinese Integrative Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 200062, China. E-mail: yinpeihao1975@hotmail.com; Fax: +86-21-52665957; Tel: +86-21-22233836
First published on 20th April 2016
Abstract
We report on the fabrication of a multifunctional polymeric prodrug covalently linked with an anticancer drug (bufalin, BUF) and tumor-targeting peptide (RGD) and investigate its anticancer performance against colon cancer in mice. The polymerizable monomer, 3-((2-(methacryloyloxy)ethyl) thio)propanoic acid (BSMA), was synthesized first. Atom radical transfer polymerization (ATRP) of BSMA and oligo(ethylene glycol) monomethyl ether methacrylate (OEGMA) afforded random copolymers, P(OEGMA-co-BSMA). The polymeric prodrug of BUF, P(OEGMA-co-BUF), was obtained by an esterification reaction between the carboxyl groups of P(OEGMA-co-BSMA) and the hydroxyl group of BUF. Finally, a tumor-targeting polymeric prodrug, P(OEGMA-co-BUF-co-RGD), was obtained via an aminolysis reaction of P(OEGMA-co-BUF) in the presence of RGD and the final drug content was determined to be ∼32.9 wt%. In aqueous media, P(OEGMA-co-BUF-co-RGD) self-assembles into micelles and the hydrodynamic diameter (Dh) of the micelles was determined to be ∼33.0 (±2.5) nm by dynamic laser light scattering (LLS). It was demonstrated that the tumor-targeting polymeric prodrug showed improved anticancer performance both in vitro and in vivo in comparison with that of free BUF.
1. Introduction
Colon cancer is one of the most incident malignant tumors and its therapy has remained a formidable challenge in the past several decades.1 As one of the most widely used tools, chemotherapy plays an important role in treating colon cancer and has attracted more and more attention in recent years. However, traditional small molecular anticancer drugs often suffer from intrinsic limitations such as poor water solubility, unsatisfactory pharmacokinetic processes (e.g., short circulation time and improper biodistribution), and severe side effects, which dramatically impact their therapeutic efficacy.2,3 For instance, bufalin (BUF), the major component of the traditional Chinese medicine Chan'su, exhibits significant activities against a broad spectrum of tumors including colon cancer.4–11 However, its poor water solubility and severe adverse effects such as high cardiac toxicity, allergic shock, ardent fever, and sinus bradycardia limit its further clinical applications.10,11To address the intrinsic limitations associated with small molecular anticancer drugs various strategies have been developed including physically encapsulating drugs into polymeric assemblies (e.g., micelles, nanogels, and vesicles) and covalently linking drugs onto polymer backbones or side chains.12–14 Among various nanocarriers, polymer-drug conjugates (PDCs) or polymeric prodrugs have received worldwide research interest as a promising drug delivery system.12–15 In polymeric prodrug systems, small molecular anticancer drugs were covalently attached onto the polymers, which can effectively overcome the shortages of nanomedicines based on non-covalent strategies such as premature drug release, low drug content, and unsatisfactory pharmacokinetic processes. Unfortunately, many traditional polymeric prodrugs were achieved via chemically stable covalent bonds, which often lead to too slow drug release and sometimes even reduced anticancer activity.
Recently, a variety of chemical strategies have been utilized to covalently anchor chemotherapeutic drugs onto the polymer backbones and/or side chains via cleavable or degradable linkages.2,16–32 Allen et al. reported an amphiphilic polyethylene glycol–docetaxel (PEG–DTX) conjugate by attaching PEG with DTX through esterase responsive β-thioester bond.15 Shen and co-workers developed a novel amphiphilic esterase-responsive prodrug by linking hydrophilic short oligomer chain of PEG (OEG) with hydrophobic anticancer drug camptothecin (CPT) through β-thioester bond.33 Amphiphilic phospholipid-mimicking prodrugs, OEG–CPT and OEG–(CPT)2 were obtained by conjugating one or two CPT molecule(s) to OEG, possessing as high as ∼40 wt% or ∼58 wt% drug content without burst drug release. Interestingly, the prodrugs released CPT quickly once inside cells, exhibiting enhanced anticancer activity both in vitro and in vivo. Wooley et al. attached paclitaxel onto block copolymers via β-thioester bond and investigated its controlled release in vitro.34 However, it should be mentioned that the biomedical application especially in vivo investigation of β-thioester bond-based prodrugs is still not fully explored although some responsive polymeric prodrugs have been synthesized.
In the current work, we tentatively introduced β-thioester bond into the design of novel polymeric prodrug of BUF with the aim of improving its anticancer performance against colon cancer (Scheme 1). Nuclear magnetic resonance (NMR), dynamic laser light scattering (LLS), and gel permeation chromatography (GPC) were employed to characterize the polymeric prodrugs and prodrug-based nanoparticles. Typical malignant colon cancer cell line LoVo cells were used to evaluate the anticancer performance of the obtained polymeric prodrugs. Flow cytometry and fluorescence imaging were employed to evaluate the capability of entering into cells for the polymeric prodrugs. Then in vivo fluorescence imaging was utilized to evaluate the biodistribution of the polymeric prodrugs. In vivo anticancer experiments were conducted to probe the feasibilities and capabilities of the polymeric prodrugs.
2. Materials and methods
2.1 Materials
Oligo(ethylene glycol) monomethyl ether methacrylate (OEGMA, Mn = 500 g mol−1, mean degree of polymerization, DP, is 8–9) purchased from Aladdin (Shanghai, China) was passed through a neutral alumina column to remove the inhibitor and then stored at −20 °C prior to use. 2-Mercaptoethanol (99%, Sigma-Aldrich) and tert-butyl acrylate (99%, Aladdin) were dried over calcium hydride (CaH2), distilled at reduced pressure, and then stored at −20 °C prior to use. Methacryloyl chloride (99%, Aladdin) was distilled at reduced pressure and then stored at −20 °C prior to use. N,N,N′,N′′,N′′-Pentamethyl diethylenetriamine (PMDETA, 98%) and copper(I) bromide (CuBr, 98%) were purchased from Sigma-Aldrich and used as received. Bufalin (BUF, 99%) was purchased from Chengdu PuRuiFa Technology Development Co. Ltd. (Chengdu, China) and used as received. Cyclic RGD (cRGD, shortened as RGD in the subsequent sections) was purchased from Chinese Peptide Company (Hangzhou, China) and used as received. Cyanine5 amine (Cy5, Lumiprobe, Florida, USA), esterase solution (porcine liver, 5 KU, Sigma-Aldrich), 4% paraformaldehyde solution (Beyotime Biotech, Shanghai, China), 4,6-diamidino-2-phenylindole (DAPI, Beyotime Biotech), cholecystokinin (CCK-8) assay kit (Beyotime Biotech), 3,3′-diamino-benzidine (Vector, Burlingame, CA, USA), and hematoxylin and eosin (H&E, Thermo Fisher Scientific, Waltham, MA, USA) were used as received. Fetal bovine serum (FBS), penicillin, streptomycin, and RPMI-1640 medium were purchased from Thermo Fisher Scientific and used as received. N,N′-Dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS, 99%), trifluoroacetic acid (TFA), 4-dimethylaminopyridine (DMAP), and all other reagents were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China) and used as received. Triethylamine (TEA) and dichloromethane (CH2Cl2) were dried over CaH2 and distilled just prior to use. Small molecular ATRP initiator (benzyl 2-bromo-2-methylpropanoate) and tert-butyl-3-((2-hydroxyethyl) thio)propanoate were synthesized according to literature procedures.35,362.2 Sample synthesis
Synthetic schemes employed for the preparation of multifunctional polymeric prodrug, P(OEGMA-co-BUF-co-RGD), covalently linked with anticancer drug (BUF) and tumor targeting peptide (RGD) are shown in Scheme 2. All NMR spectra were recorded on a Bruker AV300 NMR spectrometer (resonance frequency of 300 MHz) operated in the Fourier transform mode. CDCl3 and DMSO-d6 were used as the solvent. ESI-MS experiment was performed on Agilent 6460 Triple Quadruple mass spectrometer equipped with an ESI source, and exact masses were measured using a Thermo Scientific LTQ Orbitrap Mass Spectrometer equipped with an electrospray interface. The number-average molecular weight (Mn) and molecular-weight dispersity (ĐM = Mw/Mn, where Mw represents weight-average molecular weight) were determined by GPC equipped with Waters 1515 pump and Waters 2414 differential refractive index detector (set at 30 °C), employing a series of two linear Styragel columns (HR2 and HR4) at an oven temperature of 45 °C. The eluent was DMF at a flow rate of 1.0 mL min−1. A series of low ĐM polystyrene standards were employed for calibration.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OC(CH3)3).
O)OC(CH3)3).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–), 4.30 (2H, –OCH2CH2–), 2.80 (4H, –SCH2CH2OCO–), 2.53 (2H, –COOCH2CH2S–), 1.94 (3H, CH2
C(CH3)–), 4.30 (2H, –OCH2CH2–), 2.80 (4H, –SCH2CH2OCO–), 2.53 (2H, –COOCH2CH2S–), 1.94 (3H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–), and 1.46 (9H, –C(
C(CH3)–), and 1.46 (9H, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OC(CH3)3). 13C NMR (CDCl3, δ, ppm; Fig. S3†): 170.78 ppm, 167.30 ppm, 136.03 ppm, 125.89 ppm, 81.00 ppm, 63.80 ppm, 36.05 ppm, 30.59 ppm, 28.23 ppm, and 18.09 ppm. MS spectrum of tert-butyl-3-((2-hydroxyethyl)thio)propanoate was shown in Fig. S4a:† m/z 188 (calculated for C9H10O2S: 188).
O)OC(CH3)3). 13C NMR (CDCl3, δ, ppm; Fig. S3†): 170.78 ppm, 167.30 ppm, 136.03 ppm, 125.89 ppm, 81.00 ppm, 63.80 ppm, 36.05 ppm, 30.59 ppm, 28.23 ppm, and 18.09 ppm. MS spectrum of tert-butyl-3-((2-hydroxyethyl)thio)propanoate was shown in Fig. S4a:† m/z 188 (calculated for C9H10O2S: 188).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–), 4.30 (2H, –OCH2CH2–), 2.81–2.90 (4H, –SCH2CH2COOH), 2.70 (2H, –C(
C(CH3)–), 4.30 (2H, –OCH2CH2–), 2.81–2.90 (4H, –SCH2CH2COOH), 2.70 (2H, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OCH2CH2S–), and 1.94 (3H, CH2
O)OCH2CH2S–), and 1.94 (3H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–). 13C NMR (CDCl3, δ, ppm; Fig. S6†): 177.84 ppm, 167.27 ppm, 136.04 ppm, 126.29 ppm, 63.79 ppm, 34.49 ppm, 30.58 ppm, 26.67 ppm, and 18.08 ppm. MS spectrum of BSMA was shown in Fig. S4b:† m/z 132 (calculated for C5H8O2S: 132).
C(CH3)–). 13C NMR (CDCl3, δ, ppm; Fig. S6†): 177.84 ppm, 167.27 ppm, 136.04 ppm, 126.29 ppm, 63.79 ppm, 34.49 ppm, 30.58 ppm, 26.67 ppm, and 18.08 ppm. MS spectrum of BSMA was shown in Fig. S4b:† m/z 132 (calculated for C5H8O2S: 132).2.3 Preparation of micelles
Micelles assembled from polymeric prodrugs were prepared via the co-solvent approach. In a typical example, P(OEGMA-co-BUF-co-RGD) (10 mg) was dissolved in DMF (1 mL) and added into 9 mL deionized water under vigorous stirring. Then the solution was dialyzed against deionized water to remove organic solvent. Finally, the colloidal dispersion was diluted to the desired concentrations for further experiments.2.4 Determination of hydrodynamic diameter (Dh) and zeta potential (ζ)
Malvern Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) was used to characterize the hydrodynamic Dh and ζ of the micellar nanoparticles with 632 nm set at a scattering angle of 173°. The solution was first sonicated for ∼30 s and then the measurements were performed in disposable sizing cuvettes or zeta-potential measurement cells. Each measurement was performed in triplicate. The micelles were characterized in PBS buffer (10 mM, pH 7.4) at a concentration of 0.5 g L−1.2.5 In vitro drug release measurements
Approximately 100 μL of aqueous dispersion of drug-containing micelles (1.0 g L−1) was transferred to a dialysis cell with molecular weight cutoff of 2.0 kDa and then dialyzed against 3.4 mL of PBS buffer (pH 7.4) in the absence and presence of 10 U esterase at 37 °C. The BUF concentration in the dialysate was quantified by measuring the absorption of BUF at 298 nm by HPLC (LC-20AD Series, Shimadzu Corporation, Kyoto, Japan) against a standard calibration curve.2.6 Cell culture
The human colon cancer cell line LoVo cells were purchased from Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI 1640 medium supplemented with FBS (10%), penicillin (100 units per mL), and streptomycin (100 μg mL−1) at 37 °C humidified atmosphere with 5% CO2.2.7 In vitro cytotoxicity evaluation
LoVo cells were employed for in vitro cytotoxicity evaluation via the CCK-8 assay. Briefly, LoVo cells were first cultured for 2 days. For cytotoxicity assay, LoVo cells were seeded in a 96-well plate at an initial density of ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well in complete RPMI 1640 medium (200 μL). After incubating 24 h, 1640 medium was replaced with fresh medium, and the cells were treated with P(OEGMA-co-BSMA), free BUF, P(OEGMA-co-BUF), and P(OEGMA-co-BUF-co-RGD) at varying concentrations. The cells were then incubated for another 24 h. Subsequently, 10 μL of CCK-8 assay agents were added to the culture medium, and the cells were incubated for 1 h. Absorbance was measured at 450 nm by a microplate reader (Thermo Fisher Scientific). Each experiment condition was done in quadruple and the data are shown as the mean value plus a standard deviation (±SD).
000 cells per well in complete RPMI 1640 medium (200 μL). After incubating 24 h, 1640 medium was replaced with fresh medium, and the cells were treated with P(OEGMA-co-BSMA), free BUF, P(OEGMA-co-BUF), and P(OEGMA-co-BUF-co-RGD) at varying concentrations. The cells were then incubated for another 24 h. Subsequently, 10 μL of CCK-8 assay agents were added to the culture medium, and the cells were incubated for 1 h. Absorbance was measured at 450 nm by a microplate reader (Thermo Fisher Scientific). Each experiment condition was done in quadruple and the data are shown as the mean value plus a standard deviation (±SD).
2.8 Cellular uptake of nanoparticles via flow cytometry
LoVo cells were seeded in 24-well plates at ∼105 cells per well in 1 mL supplemented 1640 medium. After 24 h incubation, cells were treated with P(OEGMA-co-BUF-co-RGD-co-Cy5) and P(OEGMA-co-BUF-co-Cy5) at a final Cy5 concentration of 3.0 × 10−6 M for 4 h. Afterwards, the culture medium was removed and cells were washed three times with PBS and harvested with trypsin. After being fixed by 4% paraformaldehyde solution, the cells were suspended in 500 μL of PBS. The quantitative uptake was recorded by a flow cytometry (Calibur, BD, USA).2.9 Fluorescence imaging
LoVo cells were first cultured in 1640 medium at 37 °C in a CO2/air (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) incubator for 24 h. Then, LoVo cells were stained with P(OEGMA-co-BUF-co-RGD-co-Cy5) ([Cy5] = 3.0 × 10−6 M) and P(OEGMA-co-BUF-co-Cy5) ([Cy5] = 3.0 × 10−6 M) for 4 h and DAPI (the concentration of DAPI was employed according to the protocols provided by Beyotime Biotech) for 15 min at 37 °C. The cells were washed with PBS for three times and then fixed with 4% formaldehyde for 20 min at room temperature. DAPI and Cy5 were excited at 405 and 633 nm, respectively. And the fluorescence emissions were collected between 420–460 nm and 660–700 nm, respectively, by ZEISS710 confocal microscope (Carl Zeiss AG, Germany) at 37 °C. Images were taken by using the lasers sequentially with a 63× objective lens.
95) incubator for 24 h. Then, LoVo cells were stained with P(OEGMA-co-BUF-co-RGD-co-Cy5) ([Cy5] = 3.0 × 10−6 M) and P(OEGMA-co-BUF-co-Cy5) ([Cy5] = 3.0 × 10−6 M) for 4 h and DAPI (the concentration of DAPI was employed according to the protocols provided by Beyotime Biotech) for 15 min at 37 °C. The cells were washed with PBS for three times and then fixed with 4% formaldehyde for 20 min at room temperature. DAPI and Cy5 were excited at 405 and 633 nm, respectively. And the fluorescence emissions were collected between 420–460 nm and 660–700 nm, respectively, by ZEISS710 confocal microscope (Carl Zeiss AG, Germany) at 37 °C. Images were taken by using the lasers sequentially with a 63× objective lens.
2.10 Establishment of a tumor model
Subcutaneous tumors were established in nude mice (BALB/c, female, 4–6 weeks old) by injecting LoVo cells (∼5 × 106 cells in 0.2 mL PBS) into their left armpits. All experiments were carried out according to the Guidelines of the Laboratory Protocol of Animal Handling (affiliated Putuo Hospital of Shanghai University of Traditional Chinese Medicine) and approved by the Institutional Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine. Tumor size was measured using a caliper and the tumor-bearing mice were classified into six groups (n = 10): blank, free BUF, P(OEGMA-co-BSMA), P(OEGMA-co-RGD), P(OEGMA-co-BUF), and P(OEGMA-co-BUF-co-RGD).2.11 Bio-distribution of polymeric prodrugs
Fluorescent dye Cy5-labeled polymeric prodrugs, P(OEGMA-co-BUF-co-RGD-co-Cy5) and P(OEGMA-co-BUF-co-Cy5), were employed to evaluate their tumor targeting capability in LoVo colon cancer xenograft tumors. P(OEGMA-co-BUF-co-RGD-co-Cy5) (200 μL, 0.3 mg mL−1, [Cy5] = 7.9 × 10−6 M) and P(OEGMA-co-BUF-co-Cy5) (200 μL, 0.3 mg mL−1, [Cy5] = 7.9 × 10−6 M) were injected through the vena caudalis (tail vein) into the tumor-bearing xenografted mice. Fluorescence imaging of the tumor-bearing mice was performed at varying time intervals after injection by using a small animal in vivo fluorescence imaging system (LB 983; Berthold Technologies GmbH and Co KG, Bad Wildbad, Germany). After 48 h, the nude mice were sacrificed. Tumor and organs including heart, liver, spleen, lung, and kidney were collected for ex vivo distribution examination with an in vivo imaging system.2.12 In vivo study of the therapeutic efficacy in mice
The mice were classified into six groups on the basis of the solutions they were administered: normal saline (NS, 13.5 mL kg−1), P(OEGMA-co-BSMA) (24.5 mg kg−1), P(OEGMA-co-RGD) (24.5 mg kg−1), free BUF (1 mg BUF per kg), P(OEGMA-co-BUF) (1 mg BUF per kg), and P(OEGMA-co-BUF-co-RGD) (1 mg BUF per kg), by intravenous injection through the vena caudalis every 2 days. The remaining mice in each group were killed and the tumors were collected for further examination.2.13 Histological examination of tumor tissues
The removed xenograft tumors were fixed with 4% neutral buffered paraformaldehyde and embedded in paraffin before making tissue sections. 4 μm tissue sections were prepared and stained with H&E for histological examination.2.14 Immunohistochemical staining
After deparaffinization and dehydration, microwave antigen retrieval was performed for 5 min before peroxidase quenching with 3% H2O2 in PBS for 15 min. Subsequently, the sections were blocked with 5% bovine serum albumin (BSA) for 30 min and then incubated, respectively, with the primary antibodies overnight at 4 °C with a dilution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in PBS. The primary antibody anti-CD31 monoclonal antibody was used for microvessel density (MVD) analysis and anti-mouse Ki-67 monoclonal antibody was used for cell proliferation analysis. After washed with PBS, sections were then incubated with biotinylated secondary antibody for 30 min and then stained with 3,3′-diamino-benzidine for 2–5 min. Slides were counterstained with hematoxylin for 2–3 min, mounted, and examined.
100 in PBS. The primary antibody anti-CD31 monoclonal antibody was used for microvessel density (MVD) analysis and anti-mouse Ki-67 monoclonal antibody was used for cell proliferation analysis. After washed with PBS, sections were then incubated with biotinylated secondary antibody for 30 min and then stained with 3,3′-diamino-benzidine for 2–5 min. Slides were counterstained with hematoxylin for 2–3 min, mounted, and examined.
3. Results and discussion
The main aim of this study was to prepare and characterize a novel type of polymeric prodrug of BUF to enhance its anticancer performance. Typical colon cancer cell line LoVo cells were used as a model to examine its cellular uptake, biodistribution, and anticancer activity both in vitro and in vivo.3.1 Synthesis and characterization of polymeric prodrug
General routes employed for the synthesis of polymerizable monomer, BSMA, and polymeric prodrug, P(OEGMA-co-BUF-co-RGD), are shown in Scheme 2. BSMA was synthesized at first. The Michael addition reaction of tert-butyl acrylate with 2-mercaptoethanol leads to hydroxyl-containing tert-butyl-3-((2-hydroxyethyl)thio)propanoate with a yield of ∼88.2%. The structure was confirmed by 1H NMR spectrum in Fig. S1,† which was consistent with literature results.36 It was followed by the esterification reaction with an excess of methacryloyl chloride in the presence of TEA, affording 2-((3-(tert-butoxy)-3-oxopropyl)thio)ethyl methacrylate. Its structure was proved by 1H NMR, 13C NMR, and MS spectra shown in Fig. S2, S3, and S4a.† The obtained compound was hydrolyzed by TFA to afford the final compound, BSMA. The disappearance of resonance signal at ∼1.46 ppm for 1H NMR spectrum and ∼81.00 ppm for 13C NMR spectrum implied the successful hydrolysis of tert-butyl ester (Fig. S5 and S6†). And the MS spectrum of BSMA was shown in Fig. S4b.† Random copolymers, P(OEGMA-co-BSMA), were fabricated via the ATRP technique in the presence of BSMA and OEGMA using benzyl 2-bromo-2-methylpropanoate as initiator. The Mn and ĐM of P(OEGMA-co-BSMA) were determined to be 25.0 kDa and 1.29, respectively, by GPC characterization (Table S1†). The DP of P(OEGMA-co-BSMA) was calculated to be ∼72 by 1H NMR analysis (Fig. S7†). The conversion of OEGMA and BSMA were ∼65% and ∼79%, respectively, based on 1H NMR analysis of the crude product. P(OEGMA-co-BUF) was obtained by the esterification reaction between the carboxyl groups of P(OEGMA-co-BSMA) and the hydroxyl group of BUF with Mn of 37.7 kDa and ĐM of 1.21 (Table S1†). 1H NMR characterization further confirmed the successful linkage of BUF onto the polymers as shown by the appearance of characteristic peaks (peaks f, g, h, i) ascribed to BUF (Fig. S8†). Tumor-targeting polymeric prodrug, P(OEGMA-co-BUF-co-RGD), was then obtained via the reaction of P(OEGMA-co-BUF) with RGD. Typical 1H NMR peaks (j and k) of RGD indicated the successful linkage of RGD on the polymeric prodrug (Fig. S9†). GPC analysis revealed an Mn of 38.9 kDa and a ĐM of 1.32 (Table S1†). And BUF content was determined to be ∼32.9 wt%. Following similar procedures, P(OEGMA-co-BUF-co-RGD-co-Cy5) (Mn = 39.0 kDa, ĐM = 1.31; Table S1†) and P(OEGMA-co-BUF-co-Cy5) (Mn = 39.1 kDa, ĐM = 1.30; Table S1†) were obtained. Fluorescence characterization of P(OEGMA-co-BUF-co-RGD-co-Cy5) indicated a strong infrared fluorescence emission peak at around ∼665 nm (Fig. S10†).In aqueous media at pH 7.4, P(OEGMA-co-BSMA) tend to exist as unimers as shown in Fig. 1a. However, by introducing hydrophobic BUF onto the polymer backbone P(OEGAM-co-BUF) formed typical nanoparticles with intensity-average hydrodynamic Dh of ∼34.7 (±1.7) nm. And the dispersity index (μ2/Γ2) of nanoparticles was ∼0.18 (Fig. 1b). The ζ was determined to be −13.4 (±1.82) mV, exhibiting slightly negatively charged characteristics presumably due to the ionization of unreacted carboxyl groups. The Dh and μ2/Γ2 of P(OEGMA-co-BUF-co-RGD) was determined to be ∼33.0 (±2.5) nm and ∼0.15, respectively, showing little change in comparison with that of P(OEGMA-co-BUF) (Fig. 1c). The ζ was determined to be −15.0 (±1.4) mV.
 | ||
| Fig. 1 Intensity-average hydrodynamic diameter, Dh, and distribution, f(Dh), of 0.5 g L−1 (a) P(OEGMA-co-BSMA), (b) P(OEGMA-co-BUF), and (c) P(OEGMA-co-BUF-co-RGD). | ||
3.2 In vitro drug release
We then examined the in vitro drug release characteristics from micellar nanoparticles of P(OEGMA-co-BUF-co-RGD) and the results are shown in Fig. 2. Under simulated physiological condition (PBS, pH 7.4, 37 °C), only ∼20% cumulative BUF release was observed over the period of 42 h, which is in accordance with the fact that β-thioester bond is relatively stable at neutral conditions.15,33 However, upon addition of 10 U esterase which is abundant in cytosol and lysosomes approximately ∼82% cumulative release of BUF was observed over the same period.33 In the first 2 h, the BUF release rate was quite fast possibly due to the fast hydrolysis of β-thioester bond catalyzed by esterase. Whereas, after 2 h the release rate decreased gradually, this should be ascribed to the depletion of prodrugs. | ||
| Fig. 2 In vitro BUF release profiles (37 °C, 10 mM PBS, pH 7.4) from polymeric micelles of P(OEGMA-co-BUF-co-RGD) in the (a) presence and (b) absence of 10 U esterase. | ||
The relatively stable linkage between BUF and polymer backbone let the nanoparticles effectively circumvent drug burst release and premature release during circulation period. At the same time, the presence of esterase leads to fast release of BUF, endowing the nanoparticles with triggered release capability after entering into cancer cells. In addition, the introduction of tumor-targeting moiety RGD is assumed to enhance the accumulation of drugs in the cancerous tissue via active targeting effect, which will be investigated in the subsequent sections.
3.3 In vitro cytotoxicity
Typical malignant colon cancer cell line LoVo cells were employed as a model to evaluate the anticancer performance of the obtained polymeric prodrug. The polymeric carrier P(OEGMA-co-BSMA) was firstly examined. As shown in Fig. 3A, at a concentration of as high as ∼200 μg mL−1 of P(OEGMA-co-BSMA) LoVo cells still showed over 85% viability, implying the good biocompatibility of the polymer. While by treating LoVo cells with P(OEGMA-co-BUF) dramatically decreased cell viability was observed, showing improved anticancer efficacy compared with that of free BUF (Fig. 3B). The improved anticancer efficacy of P(OEGMA-co-BUF) might be caused by the fact that more than one BUF molecule can enter into cells simultaneously with the help of the nanostructure of the polymeric prodrug. It was calculated that there were ∼33.1 BUF molecules per polymer chain based on the BUF content (∼32.9%) in polymeric prodrug. In addition, it is well-recognized that one polymeric micelle is often consisted of several hundreds and even thousands polymer chains. So, it is reasonable to assume that with the internalization of one single nanoparticle more than one thousand BUF molecules entered into cells. That might explain the higher anticancer efficacy of P(OEGMA-co-BUF) than free BUF.Moreover, by introducing targeting peptide, RGD in this case, to P(OEGMA-co-BUF) further enhanced anticancer efficacy was achieved. As shown in Fig. 3B, at a BUF concentration of ∼80 nM, free BUF caused the cell viability dropped to ∼55.8%. However, when treated with P(OEGMA-co-BUF) at the same BUF dosage the cell viability decreased to ∼46.4%, lower than free BUF. And the cell viability further decreased to ∼34.1% when treated with P(OEGMA-co-BUF-co-RGD). This result implies that modifying RGD onto the polymeric prodrug might play an important role in improving the anticancer activity of BUF. In combination with literature reports about the role of RGD in drug delivery systems, the underlying mechanism might be receptor-mediated endocytosis mechanism.10,37
3.4 Cellular uptake
As described above, tumor-targeting polymeric prodrug showed improved anti-proliferation effect which was assumed partly due to active targeting to the LoVo cells. Currently, fluorescent dye Cy5-labeled polymeric prodrugs, P(OEGMA-co-BUF-co-RGD-co-Cy5) and P(OEGMA-co-BUF-co-Cy5), were employed to visualize the cellular uptake of polymeric prodrugs by confocal laser scanning microscopy. LoVo cells were cultured with nanoparticles assembled from P(OEGMA-co-BUF-co-RGD-co-Cy5) and P(OEGMA-co-BUF-co-Cy5) for 4 h, respectively, before examination. The cell nuclei stained with DAPI is blue; the red color represents nanoparticles in cells. As shown in Fig. 4, RGD modified nanoparticles, P(OEGMA-co-BUF-co-RGD-co-Cy5), exhibited much stronger fluorescence emission than that of P(OEGMA-co-BUF-co-Cy5), indicating the stronger cell entering capabilities assisted by RGD. By adding an excess of free RGD (20-fold relative to the concentration of RGD moieties of targeting prodrugs) into the culture before staining with P(OEGMA-co-BUF-co-RGD-co-Cy5), dramatic fluorescence decrease can be observed, which confirmed the importance of RGD in enhancing the cellular uptake of nanoparticles (Fig. S11†).10 Flow cytometry experiment further confirmed the result that the introduction of RGD at the hydrophilic coronas of nanoparticles dramatically facilitated the endocytosis of nanoparticles (Fig. 5).3.5 In vivo biodistribution examination
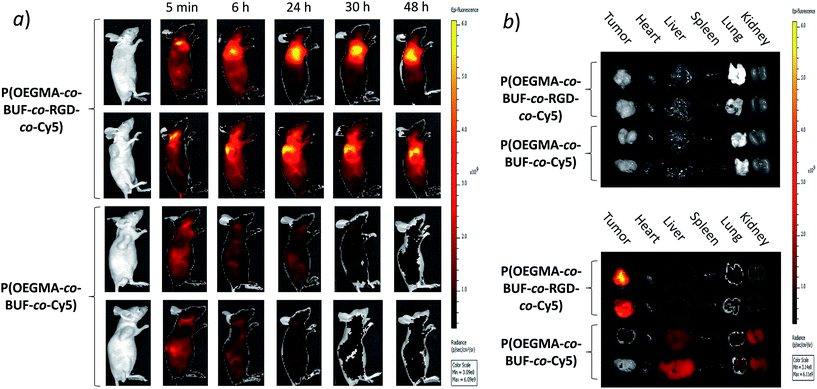
To further study the targeting efficiency of the prodrug-based nanoparticles in vivo we examined the biodistribution of Cy5-labeled P(OEGMA-co-BUF-co-RGD-co-Cy5) and P(OEGMA-co-BUF-co-Cy5) after intravenous injection. As shown in Fig. 6, two independent fluorescence imaging experiments for both P(OEGMA-co-BUF-co-RGD-co-Cy5) and P(OEGMA-co-BUF-co-Cy5) were conducted. After 5 min post injection, nanoparticles were distributed around the body and no significant difference can be observed between the two samples. However, after 6 h post injection, the fluorescence intensity in the tumor region for P(OEGMA-co-BUF-co-RGD-co-Cy5) sample was significantly stronger than that of P(OEGMA-co-BUF-co-Cy5), implying the accumulation of drugs in tumor tissues. After 24 and 30 h post injection, the fluorescence intensity in the tumor region for P(OEGMA-co-BUF-co-RGD-co-Cy5) sample became further stronger. While after 48 h post injection, the fluorescence intensity decreased slightly, possibly due to the degradation and drug release of nanoparticles. On the other hand, the fluorescence intensity in tumor region for P(OEGMA-co-BUF-co-Cy5) sample did not increase throughout the period, implying non-targeting effect for RGD-missing polymeric prodrug, P(OEGMA-co-BUF-co-Cy5). These results indicate that the introduction of RGD onto the nanoparticles resulted in better tumor targeting as well as prolonged retention time in the tumor region.3.6 In vivo anticancer activity in human colon xenograft tumors
P(OEGMA-co-BUF-co-RGD) nanoparticles showed significant improved antitumor activity in the LoVo tumor model. As illustrated in Fig. 7, P(OEGMA-co-BSMA) and P(OEGMA-co-RGD) showed no cytotoxic effect to mice as compared with control group. When the same dose of BUF was administered into the mice, the tumor inhibiting effect was much more obvious in the P(OEGMA-co-BUF) and P(OEGMA-co-BUF-co-RGD) treated groups than that of free BUF treated group. The tumor size was significantly smaller in the P(OEGMA-co-BUF-co-RGD) group than in the other groups after 4 weeks treatment probably due to increased accumulation of drug in tumor tissues via RGD-mediated active targeting and macromolecular-based EPR effects.10 This indicates the treatment efficiency on colon cancer-bearing mice is significantly enhanced via BUF conjugated polymer compared with free BUF. Besides, this therapeutic efficacy can be further improved through RGD targeting modification. | ||
| Fig. 7 Changes of the tumor size after intravenous injection of (a) saline, (b) P(OEGMA-co-BSMA), (c) P(OEGMA-co-RGD), (d) free BUF, (e) P(OEGMA-co-BUF), and (f) P(OEGMA-co-BUF-co-RGD). | ||
3.7 Histological analysis of xenograft tumors
HE-staining, MVD and Ki-67 analysis were conducted to examine the anticancer efficacy of targeting nanoparticles. As shown in Fig. 8A, tumor tissues treated with saline, P(OEGMA-co-BSMA), and P(OEGMA-co-RGD) showed relatively full and complete cytoplasm or nuclei, indicating little necrosis occurred. However, free BUF-treated tumor tissues exhibited significant nucleus pyknosis and disappearance of the cell morphology. Considering that deformation of the nucleus is a typical sign of apoptotic cells it is reasonable to say that BUF combats colon cancer via apoptosis of tumor cells.38,39 In addition, polymeric prodrugs, P(OEGMA-co-BUF) and P(OEGMA-co-BUF-co-RGD) resulted in further increased voids compared with free BUF and P(OEGMA-co-BUF-co-RGD) led to the largest void. The presence of voids within the tumor mass could be ascribed to the loss of the dead cells.40 This result was in accordance with previous in vivo anticancer results.In addition, angiogenesis in tumor tissues was examined by the MVD assay. The increase of MVD represents the growth of new microvessel in tumor tissues. As shown in Fig. 8B, large numbers of microvessel was observed in control group. And upon treated with P(OEGMA-co-BSMA) and P(OEGMA-co-RGD) the MVD of tumor tissue sections exhibited little change in comparison with that of control group. However, significant reduction in tumor MVD can be observed when treated with free BUF and polymeric prodrugs, implying that the angiogenesis of tumor can be effectively inhibited by BUF. By carefully comparing the MVD staining of the three groups, free BUF, P(OEGMA-co-BUF), and P(OEGMA-co-BUF-co-RGD), we can observe that P(OEGMA-co-BUF-co-RGD) exhibited much more effective angiogenesis inhibition effect than that of free BUF and P(OEGMA-co-BUF). This indicates that RGD-modified polymeric prodrug can effectively inhibit the angiogenesis in tumor in addition to causing cell apoptosis.
Finally, the effect of polymeric prodrugs on tumor cell proliferation was assessed by immunohistochemical staining for Ki-67. The increase of Ki-67 positive cells indicates the proliferation of tumor cells. As shown in Fig. 8C, saline, P(OEGMA-co-BSMA), and P(OEGMA-co-RGD) treated tumor resulted in a large number of Ki-67 positive cells in tumor tissue section, indicating the fast cell proliferation in tumor tissues. Reduced Ki-67 positive cells were observed in the tumor tissue section treated with free BUF. Fewer Ki-67 positive cells were observed for tumor tissue section treated with P(OEGMA-co-BUF) in comparison with that of free BUF. And further decreased Ki-67 positive cells were observed for P(OEGMA-co-BUF-co-RGD) treated tumor tissue section. Overall, tumor-targeting polymeric prodrug exhibited improved cell apoptosis, angiogenesis inhibition, and anti-proliferation effect in comparison with that of free BUF.
4. Conclusions
In summary, we fabricated a novel type of multifunctional polymeric prodrug and its anticancer performance against colon cancer both in vitro and in vivo was investigated. The employment of β-thioester bond to link BUF and the polymer backbone effectively circumvents burst/premature drug release which often exists in conventional physical encapsulation method. β-Thioester bond can be cleaved in the presence of esterase which is abundant in cytosol and endo/lysosomes, exhibiting controlled release characteristics. In addition, the introduction of targeting peptide, RGD, endows the polymeric prodrug good active targeting capability to tumor tissues in addition to passive targeting. In vitro cytotoxicity experiment showed enhanced anticancer effect for P(OEGMA-co-BUF-co-RGD). In vivo anticancer experiment demonstrated that P(OEGMA-co-BUF-co-RGD) exhibited excellent specific accumulation and prolonged retention time in tumor tissues as well as dramatically improved antitumor efficacy. Histological and immunochemical analysis demonstrated that P(OEGMA-co-BUF-co-RGD) exhibited improved cell apoptosis, angiogenesis inhibition, and anti-proliferation effect in comparison with that of free BUF. The reported tumor-targeting polymeric prodrug synergistically integrated with cancer targeted drug delivery and controlled release argues well for their potential applications as nanomedicine systems.Author contributions
Pan G, Bao YJ and Xu J contributed equally to this work; Liu T, Yin PH and Cao YJ designed the research; Pan G, Liu T, Bao YJ, Xu J, Liu C, Qiu YY, Shi XJ, Yu H, Yuan ZT, Jia TT and Yuan X performed research; Liu T wrote the paper. All authors approved the final version of the article to be published.Acknowledgements
Project supported by “Xinglin Scholar Program” of Shanghai University of Traditional Chinese Medicine (No. B-X-72), “12th Five-Year Key Discipline: integrated Chinese and western medicine and general practice training of traditional Chinese medicine” of traditional Chinese medicine of State Administration of Traditional Chinese Medicine, “Academic Leader Candidate of 315 Program” of the Health and Family Planning Commission System of Putuo District of Shanghai (No. 14Q-RC-08), and “Hospital-Supported Special Project” of Putuo Hospital of Shanghai University of Traditional Chinese Medicine (No. 2013ZD194I).References
- R. L. Siegel, K. D. Miller and A. Jemal, Ca-Cancer J. Clin., 2015, 65, 5–29 CrossRef PubMed.
- C. A. Blencowe, A. T. Russell, F. Greco, W. Hayes and D. W. Thornthwaite, Polym. Chem., 2011, 2, 773–790 RSC.
- E. Fleige, M. A. Quadir and R. Haag, Adv. Drug Delivery Rev., 2012, 64, 866–884 CrossRef CAS PubMed.
- C. Lu, K. Nan and Y. Lei, Anti-Cancer Drugs, 2008, 19, 931–939 CrossRef CAS PubMed.
- X. Cui, Y. Inagaki, H. Xu, D. Wang, F. Qi, N. Kokudo, D. Fang and W. Tang, Biol. Pharm. Bull., 2010, 33, 1728–1732 CAS.
- F. Qi, A. Li, Y. Inagaki, N. Kokudo, S. Tamura, M. Nakata and W. Tang, Int. Immunopharmacol., 2011, 11, 342–349 CrossRef CAS PubMed.
- K. Han, G. Huang, W. Gu, Y. Su, X. Huang and C. Ling, World J. Gastroenterol., 2007, 13, 3374–3379 CrossRef CAS PubMed.
- C. Yu, S. Kan, H. F. Pu, E. J. Chien and P. S. Wang, Cancer Sci., 2008, 99, 2467–2476 CrossRef CAS PubMed.
- Y. Amano, Y. Cho, M. Matsunawa, K. Komiyama and M. Makishima, J. Steroid Biochem. Mol. Biol., 2009, 114, 144–151 CrossRef CAS PubMed.
- P. Yin, Y. Wang, Y. Qiu, L. Hou, X. Liu, J. Qin, Y. Duan, P. Liu, M. Qiu and Q. Li, Int. J. Nanomed., 2012, 7, 3961–3969 CAS.
- Q. Hu, B. Liang, Y. Sun, X. L. Guo, Y. J. Bao, D. H. Xie, M. Zhou, Y. R. Duan, P. H. Yin and Z. H. Peng, Int. J. Nanomed., 2014, 9, 4035–4041 Search PubMed.
- G. Zhang, M. Zhang, J. He and P. Ni, Polym. Chem., 2013, 4, 4515–4525 RSC.
- C. Dong, W. Xia, Y. Li and T. Ren, MedChemComm, 2014, 5, 147–152 RSC.
- W. Wang, C. Li, J. Zhang, A. Dong and D. Kong, J. Mater. Chem. B, 2014, 2, 1891–1901 RSC.
- J. Liu, P. Zahedi, F. Zeng and C. Allen, J. Pharm. Sci., 2008, 97, 3274–3290 CrossRef CAS PubMed.
- E. Ko, D. Jeong, J. Kim, S. Park, G. Khang and D. Lee, Biomaterials, 2014, 35, 3895–3902 CrossRef CAS PubMed.
- E. D. Ruiz-Hernandez, M. Hess, G. J. Melen, B. Theek, M. Talelli, Y. Shi, B. Ozbakir, E. A. Teunissen, M. Ramirez, D. Moeckel, F. Kiessling, G. Storm, H. W. Scheeren, W. E. Hennink, A. A. Szalay, J. Stritzker and T. Lammers, Polym. Chem., 2014, 7, 1674–1681 RSC.
- W. Song, M. Li, Z. Tang, Q. Li, Y. Yang, H. Liu, T. Duan, H. Hong and X. Chen, Macromol. Biosci., 2012, 12, 1514–1523 CrossRef CAS PubMed.
- A. J. V. D. Vlies, U. Hasegawa and J. A. Hubbell, Mol. Pharm., 2012, 9, 2812–2818 CrossRef PubMed.
- A. N. R. Khan, J. P. Magnusson, S. Watson, A. M. Grabowska, R. W. Wilkinson, C. Alexandera and D. Pritchard, Polym. Chem., 2014, 5, 5320–5329 RSC.
- S. Lv, Z. Tang, D. Zhang, W. Song, M. Li, J. Lin, H. Liu and X. Chen, J. Controlled Release, 2014, 194, 220–227 CrossRef CAS PubMed.
- Y. Zhuang, Y. Su, Y. Peng, D. Wang, H. Deng, X. Xi, X. Zhu and Y. Lu, Biomacromolecules, 2014, 15, 1408–1418 CrossRef CAS PubMed.
- K. H. Lee, Y. J. Chung, Y. C. Kim and S. J. Song, Bull. Korean Chem. Soc., 2005, 26, 1079–1082 CrossRef CAS.
- J. Su, F. Chen, V. L. Cryns and P. B. Messersmith, J. Am. Chem. Soc., 2011, 133, 11850–11853 CrossRef CAS PubMed.
- Y. Zhang, C. Xiao, M. Li, J. Ding, C. He, X. Zhuang and X. Chen, Polym. Chem., 2014, 5, 2801–2808 RSC.
- T. Ren, W. Xia, W. Wu and Y. Li, Prog. Chem., 2013, 25, 775–784 CAS.
- R. Erez, E. Segal, K. Miller, R. S. Fainaro and D. Shabat, Bioorg. Med. Chem., 2009, 17, 4327–4335 CrossRef CAS PubMed.
- R. E. Wang, F. Costanza, Y. Niu, H. Wu, Y. Hu, W. Hang, Y. Sun and J. Cai, J. Controlled Release, 2012, 159, 154–163 CrossRef CAS PubMed.
- S. Gnaim and D. Shabat, Acc. Chem. Res., 2014, 47, 2970–2984 CrossRef CAS PubMed.
- X. Hu, J. Hu, J. Tian, Z. Ge, G. Zhang, K. Luo and S. Liu, J. Am. Chem. Soc., 2013, 135, 17617–17629 CrossRef CAS PubMed.
- X. Yang, I. Dogan, V. R. Pannala, S. Kootala, J. Hilborn and D. Ossipov, Polym. Chem., 2013, 4, 4621–4630 RSC.
- X. Hu, G. Liu, Y. Li, X. Wang and S. Liu, J. Am. Chem. Soc., 2015, 137, 362–368 CrossRef CAS PubMed.
- Y. Shen, E. Jin, B. Zhang, C. J. Murphy, M. Sui, J. Zhao, J. Wang, J. Tang, M. Fan, E. Van Kirk and W. J. Murdoch, J. Am. Chem. Soc., 2010, 132, 4259–4265 CrossRef CAS PubMed.
- J. Zou, F. Zhang, S. Zhang, S. F. Pollack, M. Elsabahy, J. Fan and K. L. Wooley, Adv. Healthcare Mater., 2014, 3, 441–448 CrossRef CAS PubMed.
- C. W. Chang, E. Bays, L. Tao, S. N. Alconcel and H. D. Maynard, Chem. Commun., 2009, 3580–3582 RSC.
- C. Boyer, G. Boutevin, J. J. Robin and B. Boutevin, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 395–415 CrossRef CAS.
- J. Park, T. F. Brust, H. J. Lee, S. C. Lee, V. J. Watts and Y. Yeo, ACS Nano, 2014, 8, 3347–3356 CrossRef CAS PubMed.
- L. Rao, D. Perez and E. White, J. Cell Biol., 1996, 135, 1441–1455 CrossRef CAS PubMed.
- E. Jin, B. Zhang, X. Sun, Z. Zhou, X. Ma, Q. Sun, J. Tang, Y. Shen, E. Van Kirk, W. J. Murdoch and M. Radosz, J. Am. Chem. Soc., 2013, 135, 933–940 CrossRef CAS PubMed.
- R. V. Kutty, S. L. Chia, M. I. Setyawati, M. S. Muthu, S. Feng and D. T. Leong, Biomaterials, 2015, 63, 58–69 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Spectroscopic/analytical data of GPC, 1H NMR, 13C NMR, MS, and fluorescence measurements. See DOI: 10.1039/c6ra05236c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |