Novel routes to quinoline derivatives from N-propargylamines
Esmail Vessally
*a,
Ladan Edjlali
b,
Akram Hosseinian
c,
Ahmadreza Bekhradnia
*d and
Mehdi D. Esrafili
e
aDepartment of Chemistry, Payame Noor University, Tehran, Iran. E-mail: vessally@yahoo.com
bDepartment of Chemistry, Tabriz Branch, Islamic Azad University, Tabriz, Iran
cDepartment of Engineering Science, College of Engineering, University of Tehran, P.O. Box 11365-4563, Tehran, Iran
dPharmaceutical Sciences Research Center, Department of Medicinal Chemistry, Mazandaran University of Medical Sciences, Sari, Iran. E-mail: reza_bnia@yahoo.com; abekhradnia@mazums.ac.ir
eDepartment of Chemistry, University of Maragheh, Maragheh, Iran
First published on 12th May 2016
Abstract
This review article is an attempt to survey literature describing synthetic methods in the preparation of quinoline derivatives from N-propargylamines. Mechanistic aspects of the reactions are considered and discussed in detail.
1 Introduction
Among bicyclic heterocycles, quinolines are the most prominent since they constitute important classes of natural products,1 synthetic pharmaceuticals,2 pesticides,3 and dyes.4 Overall, they are used as building blocks in organic synthesis,5 and electrically conducting materials.6 As a consequence, much attention has been paid to the synthesis of quinoline derivatives either by classic methods such as the Skraup,7 Friedlander,8 Doebner–Von Miller,9 and Combes10 reactions, or by transition metal-catalyzed cyclization11 and multicomponent reactions.12 However, the limited availability of starting materials, large amounts of waste, long reaction times and/or the need for expensive metal catalysts are the main drawbacks of many of these methods. In this respect, the design of improved, step-economic, atom-economic and environmentally benign approaches for their preparation is of prime importance.N-Propargylamines are versatile synthetic intermediates for many significant nitrogen-containing biologically active compounds and natural products.13 More recently, we published a review paper that covers the synthesis of highly substituted pyrroles from N-propargylamines.14 Cyclization of N-propargylamines to quinolines has emerged over the past 14 years as a powerful and novel strategy in the synthesis of the titled compounds. This new page of quinoline synthesis offers several advantages, such as: (1) shorter synthetic routes; (2) high functional group tolerance; (3) ease of handling; (4) high atom economy and many more. To the best of our knowledge, a comprehensive review has not appeared on the synthesis of quinoline derivatives from N-propargylamines in literature so far. This review is an attempt to summarize the data available from the literature about the synthesis of quinoline derivatives from N-propargylamines. The synthesis of camptothecin-family alkaloids using this new route is highlighted. Some of the important synthetic quinoline-based compounds derived from N-propargylamines are summarized in Fig. 1.
2 Quinolines from N-propargylamines
In recent years, the development of sustainable, environmentally friendly, and low cost C–C bond forming protocols have attracted much attention in synthetic organic chemistry.15 Transition metal-catalyzed hydroarylation of alkynes is a practical and atom-economy methodology that enables the insertion of a C(sp)–C(sp) bond into an aromatic C–H bond to form new C(sp2)–C(sp2) bond. The intramolecular version of this reaction has been recognized as an efficient approach to construct bicyclic aromatic frameworks.16 The intramolecular hydroarylation of allenes also is an efficient protocol for synthesis of a variety of heterocycles and has been well documented in the literature.17 The early report on the synthesis of quinolines by such a cyclization appeared in 2002, when the N-propargylamine 1 was cyclized to quinoline 2 in a one-pot reaction, using 30 mol% CuCl as the catalyst in refluxing tetrahydrofuran (Scheme 1).18Subsequently, Wang's group studied the scope of the Au-catalyzed fashion of this reaction. Thus, a variety of 2,4,6-trisubstituted quinolines 4 were synthesized via intramolecular cyclization of N-propargylamines 3 using the AuCl3/MeOH system at room temperature. It was found that the electronic character of the substituents R1 had little effect on the facility of this reaction; therefore, different functional groups such as chloride, bromide, and allyloxy can be used as substituent in the R1. However, no cyclization occurred when R2 = aryl group bearing an electron-withdrawing group, and R3 = alkyl group. According to mechanistic studies, it proceeds through the coordination of AuCl3 to the triple bond of 3, following the intramolecular nucleophilic attack of N-substituted aromatic ring onto the activated triple bond to give dihydroquinoline intermediate, which could be further oxidized by air O2 to yield quinoline derivatives 4 (Scheme 2).19 In a closely related investigation, Fu and co-workers also described that a series of trifluoromethylated N-propargylamines were converted to the corresponding 2-trifluoromethyl quinolines, via intramolecular hydroarylation of alkynes using Ph3PAuCl/AgOTf as catalytic system in toluene at 110 °C (13 examples with average yield of 84%).20
 | ||
| Scheme 2 Proposed mechanism for synthesis of quinoline derivatives 4 via Au-catalyzed hydroarylation of N-propargylamines 3. | ||
Roy and co-workers described the synthesis of a series of 2,6-disubstituted quinloines 6 via intramolecular cyclization and concomitant detosylation of easily available substrates N-aryl-N-(2-alkynyl)toluenesulfonamides 5 using 1.0 equiv. of FeCl3 in refluxing dichloromethane. The reaction scope appears that the propargylamines with electron-donating groups on the benzene ring of the terminal acetylenic part are reactive than those with electron-poor aryl groups (Scheme 3). It should be mentioned that the reaction does not work with substrates having monosubstituted alkyne moiety, due to they were unable to produce the π-complex A.21
Besides quantum dots which are used for quantitative determination of bioorganic compounds,22a–c coumarin scaffold is a privileged structure as fluorescent chemosensor.22d,e Therefore, practical approach to the synthesis of their derivatives is important. In a subsequent study, the groups of K. Litinas were able to demonstrate that a series of fused pyridocoumarins 8 could be obtained from propargylaminocoumarins 7 via hydroarylation of alkyne by treatment with Au/TiO2 in 1,2-dichloroethane at 70 °C (Scheme 4).22f
 | ||
| Scheme 4 Synthesis of fused pyridocoumarins 8 from propargylaminocoumarins 7 via hydroarylation strategy. | ||
More recently, an efficient one-pot synthesis of the 8-aminoquinoline scaffold has been developed by Schöfberger et al. they showed that 2-nitro-N-propargylanilines 9 underwent an intramolecular cyclization reaction in presence of SnCl2·2H2O or In powder in combination with hydrochloric acid as catalyst in refluxing isopropanol. The corresponding 8-aminoquinolines 10 were obtained in good yields. They probed the mechanism of the reaction and found that the reaction proceeded via a 6-endo-dig hydroarylation process. The authors examined the cyclization of the substrates having disubstituted alkyne moiety 11 under the standard reaction conditions, obtaining pyrazines 12 as the sole products in good yields. According to the proposed mechanism, this reaction is based on the 6-exo-dig hydroamination (Scheme 5).23
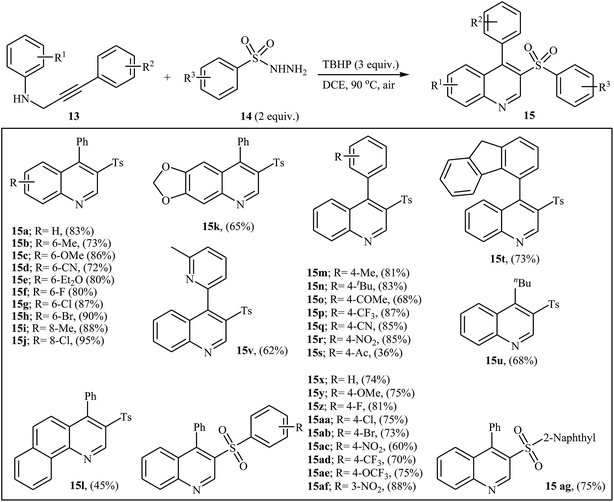
An efficient protocol for the synthesis of 3-arylsulfonylquinoline derivatives 15 via the treatment of N-propargylanilines 13 with sulfonylhydrazides 14 in the presence of tert-butyl hydroperoxide (TBHP) as oxidant in DCE at 90 °C, has been reported by Tang and co-workers in 2016. The reaction tolerates both N-propargylanilines 13 and aryl sulfonylhydrazides 14 with electron-donating and electron-withdrawing substituents and resulted in corresponding quinolines in moderate to good yields (Scheme 6). However, alkyl sulfonylhydrazides does not work in this protocol. Mechanistically, this reaction involves (Fig. 2): (1) the reaction of sulfonylhydrazide 14 with TBHP which results the formation of sulfonyl radical A; (2) intermolecular addition of A onto N-propargylaniline 13 to give alkenyl radical B; (3) intramolecular cyclization of B to furnish radical C; (4) oxidation of C to give the corresponding cyclohexadienyl cation D; (5) deprotonation of D to provide the sulfonated 1,2-dihydroquinoline E; and (6) aromatization of E to furnish final product.24
In another beautiful approach, Iqbal and co-workers disclosed a one-pot, three-component version of the same reaction where the requisite N-propargylamines were prepared in situ from aldehyde, alkyne, and amine components (A3 coupling reaction). The mechanism proposed for this transformation involves the formation of N-propargylamine intermediate C by coupling reaction of primary amine 16, aldehyde 17, and external alkyne 18. This intermediate undergoes propargyl–allenyl isomerization to form D. The formation of intermediate E occurs next, followed by its intramolecular nucleophilic attack to give a zwitterion F that, after isomerization and proton transformation, affords a more stable zwitterionic intermediate G that transforms to the final product by an oxidative process (Scheme 7).18
 | ||
| Scheme 7 Proposed mechanistic pathways for the formation of quinolines 19 via a Cu-catalyzed three-component coupling approach. | ||
One of the main drawbacks of Iqbal's method is the low yields of the desired quinolines, due to the formation of the mixture of products. To overpass this limitation, numerous catalytic system have been developed (Table 1). These include: AuCl3/CuBr,19 copper(II) triflate,25,26 FeCl3,27 montmorillonite K-10,28 CuCl-modified montmorillonite,29 HClO4-modified montmorillonite,30 YCl3,31 and Fe3O4 nanoparticles.32 A common mechanism can be proposed for all these syntheses. Following an A3 coupling of the starting materials, the reaction then proceeds along the similar mechanistic pathway that described in Scheme 2. Interestingly, when the reaction was carried out in the presence of iodine as catalyst, the target quinolines were obtained in moderate to good yields via a condensation/imino-Diels–Alder/isomerization/oxidation sequential process.33
| Entry | Catalyst (mol%) | Conditions | Number of examples | Yield (%) | |
|---|---|---|---|---|---|
| Range | Average | ||||
| 1 | AuCl3 (5) + CuBr (30) | MeOH, r.t., 96–288 h | 13 | 48–87 | 68 |
| 2 | Cu(OTf)2 (20) | DCM, r.t., 24 h | 6 | 71–83 | 77 |
| 3 | Cu(OTf)2 (5) | Neat, 100 °C, 4–48 h | 14 | 61–89 | 63 |
| 4 | FeCl3 (10) | Toluene, 110 °C, 24 h | 16 | 56–95 | 78 |
| 5 | K-10 (Mont.) | Neat, MW, 10–15 min | 27 | 56–96 | 82 |
| 6 | CuCl (30)-modified Mont. | Neat, MW, 3–8 min | 12 | 61–86 | 74 |
| 7 | HClO4 (30)-modified Mont. | Neat, 70 °C, 3–6 h | 14 | 41–81 | 58 |
| 8 | YCl3 (10) | Neat, MW, 8 min | 17 | 50–93 | 79 |
| 9 | Nano Fe3O4 (1) | Toluene, 110 °C, 3.5–6.5 h | 5 | 79–92 | 86 |
Recently, the group of Perumal has reported an efficient Sn-mediated synthesis of 2-substituted quinolines 23 via A3-coupling of 2-nitrobenzaldehydes 20, piperidine 21, and phenylacetylenes 22, followed by reductive cyclization of prepared nitro-N-benzylpropargylamines A (Scheme 8). The authors observed that, using arylamines instead of piperdine under standard reaction conditions gave exclusively 3-alkynyl-2-aryl-2H-indazoles in yields ranging from 58 to 81% instead of the expected quinolines through N–N bond formation.34
 | ||
| Scheme 8 (a) The synthesis of quinolines 23 through A3-coupling/reductive cyclization; (b) proposed mechanism for the SnCl2·2H2O mediated reductive cyclization. | ||
In a different approach, Patil and Raut have reported an interesting cascade reaction that allows the synthesis of 2-substituted quinolines 27 from 2-aminobenzaldehyde 24 and terminal alkynes 25 by the cooperative effect of two catalyst: pyrrolidine 26 and copper(I) iodide. Remarkably, a combination of both the catalysts is necessary; the use of either catalyst alone does not give the product. The mechanism shown in Scheme 9 was proposed for this process. It consists of the following key steps: formation of iminium intermediate A, reaction of intermediate A with alkyne and CuI to produce intermediate B with expulsion of water. Unification of acetylide and iminium ion in B leading to copper-coordinated propargylamine derivative C, the propargylamine C being cyclized to form intermediate D, and a protonation and aromatization to generate the desired products 27 with the liberation of CuI and pyrrolidine. Remarkably, the method has been successfully applied in synthesis of some biologically active compounds and natural products.35
 | ||
| Scheme 9 (a) Synthesis of 2-aubstituted quinolines 27 via addition/cycloisomerization cascade using cooperative catalysis; (b) proposed mechanism for cooperative catalysis. | ||
The same authors applied this cyclization to the synthesis of the 3-aminoquinolines 30 via Au-catalyzed rearrangement reaction between 2-aminobenzaldehydes 28 and N-propargylamine 29. After studying a number of solvents, such as 1,4-dioxane, methanol, acetonitrile, and toluene, and catalyst, such as CuI, Cu(OTf)2, AuCl, Ph3PAuNTt2, Ph3PAuOTf, and Ph3PAuSbF6, the system acetonitrile/Ph3PAuOTf at 50 °C was found to be optimum with respect to the yield of product isolated. The optimized protocol tolerated a variety of functional groups, such as chloro, bromo, methoxy, ester, cyano, and gave corresponding quinolines in moderate to high yields (Scheme 10).36
 | ||
| Scheme 10 Synthesis of the 3-aminoquinolines 30 via Au-catalyzed rearrangement reaction between 2-aminobenzaldehydes 28 and N-propargylamine 29. | ||
Electrophilic cyclization of alkynes has been increasingly exploited as straightforward route to the preparation of various heterocycles during the last few years since they frequently happen regioselectively under mild reaction conditions. Many electrophilic reagents such as I, Br, PhSe, PhS, etc. could induce the electrophilic cyclization of a carbon–carbon triple bond.37 Larock and co-workers were able to take advantage of this chemistry in their efforts to synthesis of a variety of 3-halogen-, selenium-, and sulfur-containing quinolines 32 by the 6-endo-dig electrophilic cyclization of N-propargylamines 31 using ICl, I2, Br2, PhSeBr, and 4-NO2-C6H4SCl as electrophiles and NaHCO3 as base in CH3CN at room temperature. Some reported examples are shown in Scheme 11. The applicability of the above cyclization product to the synthesis of more functionalized quinolines was demonstrated by transition-metal-catalyzed processes, such as Suzuki cross-coupling and Buchwald–Hartwig amination reactions.38 The same strategy was applied by Danheiser and co-workers to the synthesis of a series of fully substituted quinolines via iodocyclization of highly substituted N-propargylanilines.39
 | ||
| Scheme 11 Synthesis of 3-substituted quinolines 32 via electrophilic cyclization of N-propargylamines 31. | ||
3 Hydrogenated quinolines from N-propargylamines
The hydrogenated quinolines (dihydroquinoline and tetrahydroquinoline derivatives) are an important building class of organic compounds and widely found in many biologically active natural or synthetic products.40–49 The first general and practical approach to the synthesis of 1,2-dihydroquinolines from N-propargylamines have been reported by Williamson, March, and Ward in 1995, when 1,1-disubstitutedpropargylanilines 33 underwent an intramolecular cyclization reaction in the presence of 10 wt% CuCl in refluxing toluene to form 2,2-disubstituted-1,2-dihydroquinolines 34 (Scheme 12). The results demonstrated that the rate of cyclization was considerably influenced by electronic characters of the substituents on the aniline ring and steric effects of the substituents attached to the carbon bearing the nitrogen atom. Generally, the substrates bearing electron-withdrawing groups on the aromatic ring and bulky substituents on the C-1 position does not work well in this protocol.41,42 The same group used these dihydroquinolines as substrates in the preparation of 3-, 4- and 3,4-functionalized 2,2-dimethyl-1,2,3,4-tetrahydroquinolines via chlorination, bromination, and epoxidation of the double bond in the heterocyclic ring.43A closely related reaction that allows the preparation of N-tosylated 4-substituted 1,2-dihydroquinolines 36 was developed by the group of Komeyama, following the same strategy but starting from N-tosylated propargylanilines 35 and using Fe(OTf)3 as catalyst in DCE. Remarkably, in contrast with the Williamson's method, this reaction is tolerates electron-withdrawing substituents at the aniline ring and gave corresponding products in good to high yields. However, the substrates having electron-rich aniline rings gave poor results under this reaction conditions (Scheme 13).44
Similar intramolecular hydroarylation of N-propargylanilines using a cooperative catalytic system, consisting of FeCl3 and AgOTf were also reported by Lee and co-workers. They showed that N-propargylamines 37 and 39 underwent an intramolecular cyclization through a selective 6-endo mode in the presence of FeCl3/AgOTf as catalytic system in 1,2-dichloroethane to produce 4-(phenylthio)-1-tosyl-1,2-dihydroquinolines 38 and 1,2-dihydro-N-phenyl-N,1-ditosylquinolin-4-amines 40 in good yields, respectively (Scheme 14).45
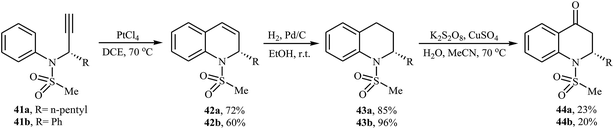
Ryu and co-workers developed the synthesis of optically active 2-substituted-2,3-dihydro-4-quinolones from N-propargylanilines via a one-pot three steps reaction. Thus, at the first step, the platinum-catalyzed hydroarylation of N-mesylpropargylanilines 41 gives 1,2-dihydroquinolines 42, which undergoes Pd-catalyzed hydrogenation into 1,2,3,4-tetrahydroquinolines 43. The formed tetrahydroquinolines 43 is converted to 2-substituted-2,3-dihydro-4-quinolones 44 via an oxidation reaction using the K2S2O8/CuSO4/H2O/MeCN system (Scheme 15).46 Previously, the same strategy was applied by the authors to the synthesis of (+)-(S)-angustureine.47
Recently, Jana and co-workers described a general and highly efficient synthesis of a wide variety of 3-acyl 1,2-dihydroquinolines 46 by the intramolecular alkyne–carbonyl metathesis of corresponding 2-(N-(prop-2-ynyl)-N-tosylamino)benzaldehydes 45 using FeCl3 as catalyst. This process was run in refluxing acetonitrile, tolerated various functional groups, and generally provided 3-acyl 1,2-dihydroquinolines 45 in good to excellent yields (Scheme 16); however, the use of a substrate with terminal alkyne failed to form the desired products. The authors also examined the cyclization of 2-aminoacetophenone derivatives.48
 | ||
| Scheme 16 Fe-catalyzed intramolecular alkyne–aldehyde metathesis for the synthesis of 3-acyl 1,2-dihydroquinolines 46. | ||
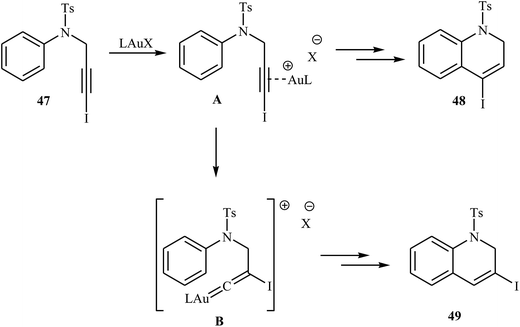
González and co-workers reported a beautiful route for construction of iodinated 1,2-dihydroquinolines 48, 49 through a gold(I)-catalyzed 5-endo-dig cyclization of N-(3-iodoprop-2-ynyl)-N-tosylaniline derivatives 47. This protocol showed different reaction behaviors depending on the nature of the substituents of the aniline ring and the electrophilic nature of the gold centre. Generally, the use of a gold complex with a phosphite ligand as catalyst, favored the direct cyclization to give the 4-iodo dihydroquinolines 48 instead of the corresponding 3-iodo dihydroquinolines 49, while by using a gold catalyst with an N-heterocyclic carbene ligand, IPrAuNTf2, the yield of 48 is decreased in favor of the 49. In the case of the nature of the substrates, the use of a substrate 47 having a more electron-rich aniline ring speeds comparatively the direct cyclization leading to 4-iodo dihydroquinolines 48, thus hampering the formation of product 49 derived from a preorganization of the system via 1,2-migration (Scheme 17).49
Two examples of 1,2-dihydro-3-iodo-4-(3-iodo-2H-chromen-4-yl)-1-tosylquinolines 51 preparation, by electrophilic intramolecular 2-fold iodoarylation of corresponding N-(6-phenoxyhexa-2,4-diynyl)benzenamines 50 in the presence of ICl/CH2Cl2 system, were recently described by Lee et al. (Scheme 18a). The author proposed mechanism for this cyclization is depicted in Scheme 18b. It should be mentioned that the products can be further functionalized by palladium-catalyzed coupling reactions.50
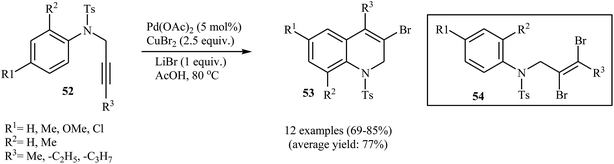
Gurunathan and Perumal have developed an efficient method to prepare 3-bromo-1,2-dihydroquinolines 53 by intramolecular cyclization of N-tosyl-N-propargyl anilines 52 in the presence of Pd(OAc)2/CuBr2 combination as catalytic system and LiBr as bromide ion source in acetic acid. The authors found that the amount of LiBr played an import role in the reaction. The best results were obtained using 1 equiv. of LiBr; if >1 equiv. was used, a mixture of dibrominated propargylamine 54 and the expected products 53 was obtained. Interestingly, when the amount of the LiBr was increased to 2 equiv. only the dibrominated product 54 was obtained as the product of the reaction. Under optimized conditions, the reaction tolerates both electron-donating and electron-withdrawing substituents at the arene ring and gave final 3-bromo-1,2-dihydroquinolines 53 in good yields (Scheme 19).51
 | ||
| Scheme 19 Pd-mediated intramolecular cyclization of N-tosyl-N-propargyl anilines 52 to quinoline 53. | ||
A one-pot reaction between indoline and alkynes provided an efficient entry into fused 1,2-dihydroquinolines 59, as shown by Che and co-workers. This transformation was carried out in nitromethane using the gold complex 60/AgSbF6 as catalytic system, and afforded fused dihydroquinolines 59 in good to high yields. The mechanistic course of this reaction sequence is shown in Scheme 20, and involves the initial formation of the enamine intermediate 57 from the starting indoline 55 and one molecule of alkyne 56. The reaction of intermediate 57 with another alkyne affords the propargylamine intermediate 58, which then undergoes an intramolecular hydroarylation to produce the observed 1,2-dihydroquinolines 59.52
Recently, Yu et al. developed a concise synthetic route to 5-tosyl-6,7-dihydro-5H-indeno[2,1-c]quinolines 63 from the reaction of propargylanilines 61 with aromatic aldehyde acetals 57 through a Fe(III)-catalyzed tandem carboarylation/cyclization in moderate to good yields (Scheme 21). The reaction is tolerant toward a variety of functional groups such as chloride, fluoride and alkoxide on the aryl groups of the NAr moieties. This made possible the further derivatization of the products. However, the reaction does not work well with the acetals of the heterocyclic aromatic aldehydes and the propargylanilines bearing electron-poor aryl rings in the alkyne terminus. The interesting result is that when a high catalyst loading (300%) is used, instead of 5-tosyl-6,7-dihydro-5H-indeno[2,1-c]quinolines 63, 7H-indeno[2,1-c]quinoline derivatives are formed.53
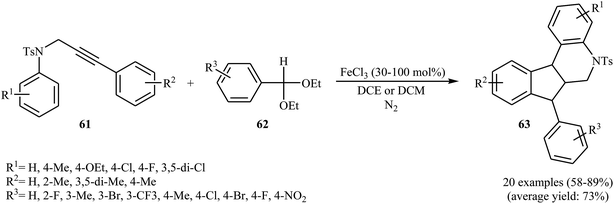 | ||
| Scheme 21 Synthesis of indeno[2,1-c]quinolines 63 via Fe-mediated carboarylation/cyclization of N-propargylanilines 61 with acetals 62. | ||
4 Camptothecin-family alkaloids from N-propargylamines
Quinoline alkaloids are of great interest to medicinal chemistry due to their broad range of biological activity.54 Camptothecin-family alkaloids (pyrrolo[3,4-b]quinoline-based alkaloids) have attracted significant interest due to their intriguing structures and biological activity (Fig. 3).55 Luotonin A is an important member of this family that first isolated in 1997 from the plant Peganum nigellastrum56 and it has shown promising cytotoxicities towards selected human cancer cell lines.57 In this regard, the synthesis of this alkaloid has undergone an explosive growth in recent years.58The early report on the synthesis of luotonins from N-propargylamines appeared in 2004, when N-propargyl quinazolinone aldehyde 66 was cyclized to luotonin A via intramolecular Povarov reaction with aniline 67 in the presence of dysprosium(III) triflate in acetonitrile (Scheme 22a). The authors applied this methodology to the synthesis of camptothecin and topotecan precursor 70 (Scheme 22b).59
In a closely related investigation, Yao and co-workers also found that N-propargyl quinazolinone amide 71 was converted to the corresponding luotonin A, via intramolecular Povarov reaction using triphenylphosphine oxide in the presence of (CF3SO2)2O at room temperature (Scheme 23a).60,61 Subsequently, N. Haider and S. Nuß studied the scope of this reaction. They found that the reaction tolerated many functional groups, including methoxy, chloro, cyano, and nitro. However, the reaction does not work well with the substrates having a strongly electron-withdrawing and solubility-decreasing group at the aniline ring (Scheme 23b).62
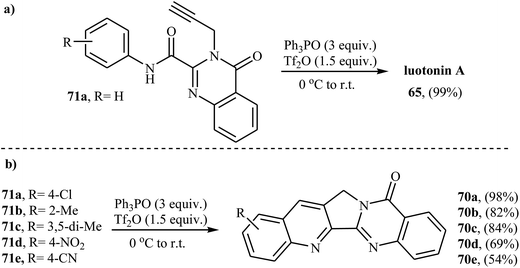 | ||
| Scheme 23 Synthesis of luotonin A derivatives 65 by intramolecular cycloaddition of N-propargylamines 71. | ||
Along this line, Chu's group described an efficient Yb-catalyzed one-pot synthesis of luotonin A and its derivatives, starting from a 2-aminobenzamide 73; which was prepared from the reaction of commercially available isatoic anhydride 72 with N-propargylamine 31. As shown in Scheme 24, the reaction of 73 with glyoxal and anilines 74 in the presence of a Lewis acid yielded the imines 76 which underwent cyclization, followed by an intramolecular aza-Diels–Alder reaction/dehydrogenation/aromatization subsequent process afforded the desired structure 77.63
 | ||
| Scheme 24 Yb-catalyzed one-pot synthesis of luotonin A and its derivatives 77 from N-propargylamine 73. | ||
Dai, Petersen, and Wang developed an efficient DBU-catalyzed synthesis of substituted ABCD ring cores of the camptothecin family of alkaloids 79 from 1,6-dihydro-6-oxo-(3-phenyl-2-propynyl)-2-pyridinecarbonitrile derivatives 78 in 1,2-dichlorobenzene at 110 °C (Scheme 25). According to the proposed mechanism, the reaction proceeds in three consecutive steps: (a) formation of an allenic intermediate A through 1,3-prototropic rearrangement of the corresponding propargylamine, (b) intramolecular hetero Diels–Alder reaction between nitrile and allen group to form intermediate B, and (c) aromatization of B to the expected product 78.64
More recently, Arumugam and co-workers described the synthesis of pyrrolo[3,4-b]quinolines 82 via a Lewis acid catalyzed sequential reaction of N-propargyl aldehyde 80 with anilines 81 under mild reaction conditions. Several catalysts and solvents were tested, and the system BF3·OEt2/CH2Cl2 was found to be superior. It is worth noting that the electronic character of anilines had very little effect on the facility of reaction. Under optimized conditions, the reaction tolerates electron-nature, electron-donating and electron-withdrawing substituents at anilines and gave the corresponding pyrrolo[3,4-b]quinolines 82 in excellent yields. According to the proposed mechanism, the reaction proceeded via a condensation/cyclization/aromatization sequential process (Scheme 26).65
 | ||
| Scheme 26 Proposed mechanism for the formation of pyrrolo-[3,4-b]quinolines 82 from treatment of N-propargylamines 80 with anilines 81. | ||
5 Summary and outlook
As a result of growing interest in the efficient protocols for the synthesis of quinolines and their derivatives, due to their major role in drug discovery, N-propargylamines have aroused interest as highly versatile and efficient reagent for preparation of titled compounds. They have been successfully transformed into substituted quinolines, dihydroquinolines, tetrahydroquinolines, and camptothecin-family alkaloids. In many cases, the use of this new page of quinoline synthesis offers several advantages over more conventional methodologies, which can be summarized as follows: (1) shorter synthetic routes; (2) high functional group tolerance; (3) ease of handling; (4) high atom economy; (5) high yielding, wide in scope and many more. Needless to say, the vast majority of the used references in this review are less than ten years old, which shows how young and vibrant this area of research is. We conclude this review by hoping that it will stimulate researchers to develop the highly versatile and extremely effective procedures to synthesis of quinolines and their ring fused analogues from titled compounds.Acknowledgements
Payame Noor University is acknowledged for Financial Support.References
- (a) M. Isobe, T. Nishikawa, N. Yamamoto, T. Tsukiyama, A. Ino and T. Okita, J. Heterocycl. Chem., 1992, 29, 619–625 CrossRef CAS; (b) J. P. Michael, Nat. Prod. Rep., 1997, 14, 605–618 RSC; (c) Z.-Z. Ma, Y. Hano, T. Nomura and Y.-J. Chen, Heterocycles, 1999, 8, 1883–1889 Search PubMed; (d) Z.-Z. Ma, Y. Hano, T. Nomura and Y.-J. Chen, Phytochemicals, 2000, 53, 1075–1078 CrossRef CAS.
- A. Marella, O. P. Tanwar, R. Saha, M. R. Ali, S. Srivastava, M. Akhter, M. Shaquiquzzaman and M. M. Alam, Saudi Pharm. J., 2013, 21, 1–12 CrossRef PubMed.
- (a) M. Xu, T. Wagerle, J. K. Long, G. P. Lahm, J. D. Barry and R. M. Smith, Bioorg. Med. Chem. Lett., 2014, 24, 4026–4030 CrossRef CAS PubMed; (b) J.-F. Tian, J. Liu, X.-F. Sun, B.-S. Chai and C.-L. Liu, Agrochemicals, 2011, vol. 8, p. 003 Search PubMed.
- (a) P. l. Lam, C. w. Kan, M. C. w. Yuen, S. y. Cheung, R. Gambari, K. h. Lam, J. C. o. Tang and C. h. Chui, Color. Technol., 2012, 128, 192–198 CrossRef CAS; (b) M. Mao, X. Zhang, B. Zhu, J. Wang, G. Wu, Y. Yin and Q. Song, Dyes Pigm., 2016, 124, 72–81 CrossRef CAS; (c) B. N. Mongal, A. Pal, T. K. Mandal, J. Datta and S. Naskar, Polyhedron, 2015, 102, 615–626 CrossRef CAS.
- (a) M. Rueping, A. P. Antonchick and T. Theissmann, Angew. Chem., Int. Ed., 2006, 45, 3683–3686 CrossRef CAS PubMed; (b) G. Franciò, F. Faraone and W. Leitner, Angew. Chem., Int. Ed., 2000, 39, 1428–1430 CrossRef.
- (a) J. I. Kim, I.-S. Shin, H. Kim and J.-K. Lee, J. Am. Chem. Soc., 2005, 127, 1614–1615 CrossRef CAS PubMed; (b) A. Bakhshi and G. Bhalla, J. Sci. Ind. Res., 2004, 63, 715–728 CAS.
- Z. H. Skraup, Ber. Dtsch. Chem. Ges., 1880, 13, 2086–2087 Search PubMed.
- P. Friedlander, Ber. Dtsch. Chem. Ges., 1882, 15, 2572–2575 CrossRef.
- O. Doebner and W. von Miller, Ber. Dtsch. Chem. Ges., 1881, 14, 2812–2817 CrossRef.
- A. Combes, Bull. Soc. Chim. Fr., 1888, 49, 89 Search PubMed.
- (a) C. S. Cho, J. S. Kim, B. H. Oh, T.-J. Kim, S. C. Shim and N. S. Yoon, Tetrahedron, 2000, 56, 7747–7750 CrossRef CAS; (b) D. K. O'Del and K. M. Nicholas, J. Org. Chem., 2003, 68, 6427–6430 CrossRef PubMed.
- (a) T. Demaude, L. Knerr and P. Pasau, J. Comb. Chem., 2004, 6, 768–775 CrossRef CAS PubMed; (b) S.-L. Zhu, K. Zhao, X.-M. Su and S.-J. Ji, Synth. Commun., 2009, 39, 1355–1366 CrossRef CAS; (c) A. Kulkarni and B. Török, Green Chem., 2010, 12, 875–878 RSC.
- (a) F. J. Fananás, T. Arto, A. Mendoza and F. Rodriguez, Org. Lett., 2011, 13, 4184–4187 CrossRef PubMed; (b) T. P. Lebold, A. B. Leduc and M. A. Kerr, Org. Lett., 2009, 11, 3770–3772 CrossRef CAS PubMed; (c) A. Arcadi, S. Cacchi, L. Cascia, G. Fabrizi and F. Marinelli, Org. Lett., 2001, 3, 2501–2504 CrossRef CAS PubMed; (d) Z. Jiang, P. Lu and Y. Wang, Org. Lett., 2012, 14, 6266–6269 CrossRef CAS PubMed.
- E. Vessally, RSC Adv., 2016, 6, 18619–18631 RSC.
- (a) B. List, R. A. Lerner and C. F. Barbas, J. Am. Chem. Soc., 2000, 122, 2395–2396 CrossRef CAS; (b) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215–1292 CrossRef CAS PubMed; (c) L. Zhao and C. J. Li, Angew. Chem., Int. Ed., 2008, 47, 7075–7078 CrossRef CAS PubMed; (d) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068–5083 RSC.
- (a) D. J. Schipper, M. Hutchinson and K. Fagnou, J. Am. Chem. Soc., 2010, 132, 6910–6911 CrossRef CAS PubMed; (b) Y.-L. Wang, W.-M. Zhang, J.-J. Dai, Y.-S. Feng and H.-J. Xu, RSC Adv., 2014, 4, 61706–61710 RSC; (c) W. Zhou, Y. Yang, Z. Wang and G.-J. Deng, Org. Biomol. Chem., 2014, 12, 251–254 RSC; (d) L. Alonso-Marañón, M. M. Martínez, L. A. Sarandeses and J. P. Sestelo, Org. Biomol. Chem., 2015, 13, 379–387 RSC; (e) J. Yun, J. Park, J. Kim and K. Lee, Tetrahedron Lett., 2015, 56, 1045–1048 CrossRef CAS.
- (a) R. M. Zeldin and F. D. Toste, Chem. Sci., 2011, 2, 1706–1709 RSC; (b) B. Alcaide, P. Almendros, S. Cembellín, T. M. del Campo and I. Fernández, Chem. Commun., 2013, 49, 1282–1284 RSC; (c) A. Fazeli, D. Pflästerer, M. Rudolph and A. S. K. Hashmi, J. Organomet. Chem., 2015, 795, 68–70 CrossRef CAS.
- H. S. Huma, R. Halder, S. S. Kalra, J. Das and J. Iqbal, Tetrahedron Lett., 2002, 43, 6485–6488 CrossRef.
- F. Xiao, Y. Chen, Y. Liu and J. Wang, Tetrahedron, 2008, 64, 2755–2761 CrossRef CAS.
- M. Zhu, W. Fu, G. Zou, C. Xun, D. Deng and B. Ji, J. Fluorine Chem., 2012, 135, 195–199 CrossRef CAS.
- (a) B. Roy, I. Ansary, S. Samanta and K. Majumdar, Tetrahedron Lett., 2012, 53, 5119–5122 CrossRef CAS; (b) A. Shafiee, M. Z. Kassaee and A. R. Bekhradnia, J. Heterocycl. Chem., 2007, 44, 471–474 CrossRef CAS.
- (a) S. N. Azizi, P. Shakeri, M. J. Chaichi, A. Bekhradnia, M. Taghavi and M. Ghaemy, Spectrochim. Acta, Part A, 2014, 122, 482–488 CrossRef CAS PubMed; (b) S. N. Azizi, M. J. Chaichi, P. Shakeri and A. Bekhradnia, J. Fluoresc., 2013, 23, 227–235 CrossRef CAS PubMed; (c) S. N. Azizi, M. J. Chaichi, P. Shakeri and A. Bekhradnia, J. Lumin., 2013, 144, 34–40 CrossRef CAS; (d) T. Zhu, Y. Wang, W. Ding, J. Xu and R. Chen, et al., Chem. Biol. Drug Des., 2015, 85, 385–393 CrossRef CAS PubMed; (e) A. Bekhradnia, E. Domehri and M. Khosravi, Spectrochim. Acta, Part A, 2016, 152, 18–22 CrossRef CAS PubMed; (f) T. S. Symeonidis, I. N. Lykakis and K. E. Litinas, Tetrahedron, 2013, 69, 4612–4616 CrossRef CAS.
- S. Aichhorn, M. Himmelsbach and W. Schöfberger, Org. Biomol. Chem., 2015, 13, 9373–9380 CAS.
- L. Zhang, S. Chen, Y. Gao, P. Zhang, Y. Wu, G. Tang and Y. Zhao, Org. Lett., 2016, 18, 1286–1289 CrossRef CAS PubMed.
- H. Huang, H. Jiang, K. Chen and H. Liu, J. Org. Chem., 2009, 74, 5476–5480 CrossRef CAS PubMed.
- C. E. Meyet and C. H. Larsen, J. Org. Chem., 2014, 79, 9835–9841 CrossRef CAS PubMed.
- K. Cao, F. M. Zhang, Y. Q. Tu, X. T. Zhuo and C. A. Fan, Chem.–Eur. J., 2009, 15, 6332–6334 CrossRef CAS PubMed.
- A. Kulkarni and B. Török, Green Chem., 2010, 12, 875–878 RSC.
- X.-L. Chen, J.-M. Zhang, W.-L. Shang, B.-Q. Lu and J.-A. Jin, J. Fluorine Chem., 2012, 133, 139–145 CrossRef CAS.
- S. K. Guchhait, K. Jadeja and C. Madaan, Tetrahedron Lett., 2009, 50, 6861–6865 CrossRef CAS.
- L. Zhang, B. Wu, Y. Zhou, J. Xia, S. Zhou and S. Wang, Chin. J. Chem., 2013, 31, 465–471 CrossRef CAS.
- S. Kaur, M. Kumar and V. Bhalla, Chem. Commun., 2015, 51, 16327–16330 RSC.
- X. Li, Z. Mao, Y. Wang, W. Chen and X. Lin, Tetrahedron, 2011, 67, 3858–3862 CrossRef CAS.
- N. Sudhapriya, A. Nandakumar and P. T. Perumal, RSC Adv., 2014, 4, 58476–58480 RSC.
- N. T. Patil and V. S. Raut, J. Org. Chem., 2010, 75, 6961–6964 CrossRef CAS PubMed.
- N. T. Patil, V. S. Raut, V. S. Shinde, G. Gayatri and G. N. Sastry, Chem.–Eur. J., 2012, 18, 5530–5535 CrossRef CAS PubMed.
- B. Godoi, R. F. Schumacher and G. Zeni, Chem. Rev., 2011, 111, 2937–2980 CrossRef CAS PubMed.
- X. Zhang, T. Yao, M. A. Campo and R. C. Larock, Tetrahedron, 2010, 66, 1177–1187 CrossRef CAS PubMed.
- T. P. Willumstad, P. D. Boudreau and R. L. Danheiser, J. Org. Chem., 2015, 80, 11794–11805 CrossRef CAS PubMed.
- (a) M. M. Ghorab, F. A. Ragab and M. M. Hamed, Eur. J. Org. Chem., 2009, 44, 4211–4217 CAS; (b) H. C. Smith, C. K. Cavanaugh, J. L. Friz, C. S. Thompson, J. A. Saggers, E. L. Michelotti, J. Garcia and C. M. Tice, Bioorg. Med. Chem. Lett., 2003, 13, 1943–1946 CrossRef CAS PubMed; (c) S. Tu, X. Zhu, J. Zhang, J. Xu, Y. Zhang, Q. Wang, R. Jia, B. Jiang, J. Zhang and C. Yao, Bioorg. Med. Chem. Lett., 2006, 16, 2925–2928 CrossRef CAS PubMed; (d) M. L. Quan, P. C. Wong, C. Wang, F. Woerner, J. M. Smallheer, F. A. Barbera, J. M. Bozarth, R. L. Brown, M. R. Harpel and J. M. Luettgen, J. Med. Chem., 2014, 57, 955–969 CrossRef CAS PubMed; (e) R. J. Brideau, M. L. Knechtel, A. Huang, V. A. Vaillancourt, E. E. Vera, N. L. Oien, T. A. Hopkins, J. L. Wieber, K. F. Wilkinson and B. D. Rush, Antiviral Res., 2002, 54, 19–28 CrossRef CAS PubMed; (f) E. Stern, G. G. Muccioli, B. Bosier, L. Hamtiaux, R. Millet, J. H. Poupaert, J.-P. Hénichart, P. Depreux, J.-F. Goossens and D. M. Lambert, J. Med. Chem., 2007, 50, 5471–5484 CrossRef CAS PubMed; (g) L. Zhi, C. M. Tegley, K. B. Marschke, D. E. Mais and T. K. Jones, J. Med. Chem., 1999, 42, 1466–1472 CrossRef CAS PubMed; (h) I. Ukrainets, L. Sidorenko, O. Gorokhova and N. Jaradat, Chem. Heterocycl. Compd., 2006, 42, 475–487 CrossRef CAS.
- N. M. Williamson, D. R. March and A. D. Ward, Tetrahedron Lett., 1995, 36, 7721–7724 CrossRef CAS.
- M. A. Holman, N. M. Williamson and A. D. Ward, Aust. J. Chem., 2005, 58, 368–374 CrossRef CAS.
- N. M. Williamson and A. D. Ward, Tetrahedron, 2005, 61, 155–165 CrossRef CAS.
- K. Komeyama, R. Igawa and K. Takaki, Chem. Commun., 2010, 46, 1748–1750 RSC.
- D. Eom, J. Mo, P. H. Lee, Z. Gao and S. Kim, Eur. J. Org. Chem., 2013, 2013, 533–540 CrossRef CAS.
- S. Choi, K. Jung and J. Ryu, Arch. Pharmacal Res., 2006, 29, 369–374 CrossRef CAS.
- J. Ryu, Bull. Korean Chem. Soc., 2006, 27, 631–632 CrossRef CAS.
- S. Jalal, K. Bera, S. Sarkar, K. Paul and U. Jana, Org. Biomol. Chem., 2014, 12, 1759–1770 CAS.
- P. Morán-Poladura, S. Suárez-Pantiga, M. Piedrafita, E. Rubio and J. M. González, J. Organomet. Chem., 2011, 696, 12–15 CrossRef.
- J. Mo, W. Choi, J. Min, C.-E. Kim, D. Eom, S. H. Kim and P. H. Lee, J. Org. Chem., 2013, 78, 11382–11388 CrossRef CAS PubMed.
- S. Gurunathan and P. T. Perumal, Tetrahedron Lett., 2011, 52, 1783–1787 CrossRef CAS.
- X.-Y. Liu, P. Ding, J.-S. Huang and C.-M. Che, Org. Lett., 2007, 9, 2645–2648 CrossRef CAS PubMed.
- Q. Yang, T. Xu and Z. Yu, Org. Lett., 2014, 16, 6310–6313 CrossRef CAS PubMed.
- (a) K.-H. Lam, K. K.-H. Lee, R. Gambari, S. H.-L. Kok, T.-W. Kok, A. S.-C. Chan, Z.-X. Bian, W.-Y. Wong, R. S.-M. Wong and F.-Y. Lau, Phytomedicine, 2014, 21, 877–882 CrossRef CAS PubMed; (b) P.-Y. Chung, Z.-X. Bian, H.-Y. Pun, D. Chan, A. S.-C. Chan, C.-H. Chui, J. C.-O. Tang and K.-H. Lam, Future Med. Chem., 2015, 7, 947–967 CrossRef CAS PubMed; (c) R. Kaur and S. Arora, J. Crit. Rev., 2015, 2, 1–8 Search PubMed; (d) Z.-d. Yang, D.-b. Zhang, J. Ren and M.-j. Yang, Med. Chem. Res., 2012, 21, 722–725 CrossRef CAS.
- W. Du, Tetrahedron, 2003, 59, 8649–8687 CrossRef CAS.
- Z.-Z. Ma, Y. Hano, T. Nomura and Y.-J. Chen, Heterocycles, 1997, 541–546 CAS.
- (a) M. Z. Kassaee and A. R. Bekhradnia, J. Biosci. Bioeng., 2003, 95, 526–529 CrossRef CAS PubMed; (b) A. Cagir, S. H. Jones, R. Gao, B. M. Eisenhauer and S. M. Hecht, J. Am. Chem. Soc., 2003, 125, 13628–13629 CrossRef CAS PubMed.
- (a) T. R. Kelly, S. Chamberland and R. A. Silva, Tetrahedron Lett., 1999, 40, 2723–2724 CrossRef CAS; (b) J. Yadav and B. Reddy, Tetrahedron Lett., 2002, 43, 1905–1907 CrossRef CAS; (c) S. Dallavalle, L. Merlini, G. L. Beretta, S. Tinelli and F. Zunino, Bioorg. Med. Chem. Lett., 2004, 14, 5757–5761 CrossRef CAS PubMed; (d) S. P. Chavan and R. Sivappa, Tetrahedron, 2004, 60, 9931–9935 CrossRef CAS; (e) A. Servais, M. Azzouz, D. Lopes, C. Courillon and M. Malacria, Angew. Chem., Int. Ed., 2007, 46, 576–579 CrossRef CAS PubMed; (f) Y. Ju, F. Liu and C. Li, Org. Lett., 2009, 11, 3582–3585 CrossRef CAS PubMed; (g) Y.-p. Zhu, Z. Fei, M.-c. Liu, F.-c. Jia and A.-x. Wu, Org. Lett., 2012, 15, 378–381 CrossRef PubMed.
- H. Twin and R. A. Batey, Org. Lett., 2004, 6, 4913–4916 CrossRef CAS PubMed.
- H.-B. Zhou, G.-S. Liu and Z.-J. Yao, J. Org. Chem., 2007, 72, 6270–6272 CrossRef CAS PubMed.
- H.-B. Zhou, G.-S. Liu and Z.-J. Yao, Org. Lett., 2007, 9, 2003–2006 CrossRef CAS PubMed.
- N. Haider and S. Nuß, Molecules, 2012, 17, 11363–11378 CrossRef CAS PubMed.
- M.-C. Tseng, Y.-W. Chu, H.-P. Tsai, C.-M. Lin, J. Hwang and Y.-H. Chu, Org. Lett., 2011, 13, 920–923 CrossRef CAS PubMed.
- W. Dai, J. L. Petersen and K. K. Wang, Org. Lett., 2006, 8, 4665–4667 CrossRef CAS PubMed.
- A. I. Almansour, N. Arumugam, R. S. Kumar, J. C. Menéndez, H. A. Ghabbour, H.-K. Fun and R. R. Kumar, Tetrahedron Lett., 2015, 56, 6900–6903 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |