Molecular logic gates based on DNA tweezers responsive to multiplex restriction endonucleases†
Abstract
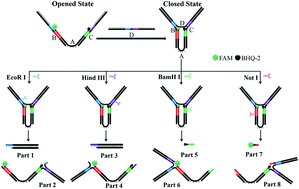
DNA logic gates have received significant attention as biocompatible building blocks of molecular circuits. Herein, we described the construction of DNA self-assembled molecular tweezers containing four different restriction endonuclease recognition sites and application of the tweezers in the construction of DNA logic gates. An open tweezer is formed by three DNA oligonucleotides, one of which is labelled with a fluorophore and a quencher at the two ends. Addition of the fourth oligonucleotide might close the tweezers, thus turning off the fluorescence signal. The quenched fluorescence of the closed tweezers can be turned on again by any one of the four restriction endonucleases, thus conferring the tweezers with the ability for multiplex detection of the four endonucleases. Based on the fluorescence responses to DNA oligonucleotide and restriction endonucleases, a set of DNA logic gates, including one-input NOT and YES logic gates, two-input IMPLICATION logic gates, two, three and four-input OR logic gates, were constructed.

 Please wait while we load your content...
Please wait while we load your content...