Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel photo-curable polyurethane resin for stereolithography†
Le Hoang
Sinh‡
ab,
Korhonen
Harri‡
a,
Liikanen
Marjo
a,
Malin
Minna
a,
Nguyen Dang
Luong
a,
Weisser
Jürgen
c,
Walter
Torsten
c,
Schnabelrauch
Matthias
c and
Seppälä
Jukka
*a
aLaboratory of Polymer Technology, Department of Biotechnology and Chemical Technology, Aalto University, School of Chemical Technology, P. O. Box 16100, FI-00076 Aalto, Finland. E-mail: jukka.seppala@aalto.fi
bCenter for Advanced Chemistry, Institute of Research and Development, Duy Tan University, Da Nang, Vietnam
cInnovent e.V., Biomaterials Department, Jena, Germany
First published on 19th May 2016
Abstract
A novel photo-curable polyurethane resin for stereolithography has been demonstrated herein. The resin was printed by stereolithography to form 3-dimensional elastomeric objects without need of any diluent. The resultant elastomer exhibited good mechanical strength, high elasticity, and excellent cell adhesion and proliferation.
Implanted devices that are in contact with the human body have improved the quality of life of many people that have lost a body part or its function.1–3 Besides the choice of materials with high biostability and biocompatibility, the architecture of the implanted devices is also an important factor. In the last decade, solid freeform fabrication (SFF) methods have increasingly been applied in creating small implanted devices with complex structures in a fast way.4 Common and well-established SFF technologies are stereolithography, selective laser sintering, three-dimensional (3D) printing and fused deposition modelling.5–7 Among these techniques, stereolithography is the most versatile method with the highest accuracy and precision.8 Its working principle is based on spatially controlled solidification of a liquid photo-curable resin. Using a computer-controlled laser beam or a digital light projector with a computer-driven building stage, a solid 3D object can be constructed in a layer-by-layer fashion. The possible use of scanning data from imaging technologies, such as magnetic resonance imaging (MRI) and tomography techniques enable stereolithography to be used to fabricate patient-specific models or implants.6,9,10 Unfortunately, the limited number of commercially available resins for processing by stereolithography has often been considered as the main limitation of the technique.11 The resin should be a liquid that rapidly solidifies upon illumination with light. Most of the available stereolithography resins are based on low-molecular weight, multi-functional monomers, and highly crosslinked networks.12 These materials are predominantly glassy, rigid and brittle. Only few resins have been described that allow the preparation of elastomeric objects by stereolithography. These resin formulations include macromers with low glass transition and relative high molecular weight (e.g. 1–5 kDa).13,14 However, they are highly viscous liquid or solid, and thus, usually required a non-reactive diluent such as N-methylpyrrolidone (NMP) or water to reduce the viscosity of the resin. This causes shrinking and internal stress of the printed objects and can reduce the accuracy or/and destroy the architecture of the objects.12–14 Thus, there is a need of elastic materials with high biocompatibility that could be used in stereolithography for medical applications.
Herein, we propose a novel photo-curable polyurethane resin for stereolithography.15 The synthesis procedure for polyurethane resin is presented in Scheme 1. In particular, isophorone diisocyanate (IPDI) was dissolved in anhydrous tetrahydrofuran (THF). A prepared mixture of hydroxyl-terminated poly(dimethylsiloxane) (PDMS, Mn 550 g mol−1) and poly(tetrahydrofuran) (PTH, Mn 2000 g mol−1) in THF was added slowly to make sure that the concentration of polyols was substantially lower than the concentration of the isocyanate. This eliminated the formation of high molecular weight resin. The molar ratio of PTH and PDMS was optimized to get proper backbone flexibility of the resin. The reaction was carried out for 1 hour. Then, a prepared mixture of 2-hydroxyethyl methacrylate and 2-methyl-1-propanol was added rapidly to the reaction mixture and the reaction was continued for another 2.5 hours. Next, 2-methyl-1-propanol was used to control the methacrylate functionality of polyurethane resin. Finally, an excess amount of 2-methyl-1-propanol was added to stop the reaction (for more details see Experimental section, ESI†). In this work, an aliphatic isocyanate, isophorone diisocyanate, was chosen due to its better biocompatibility in comparison with aromatic isocyanates.16
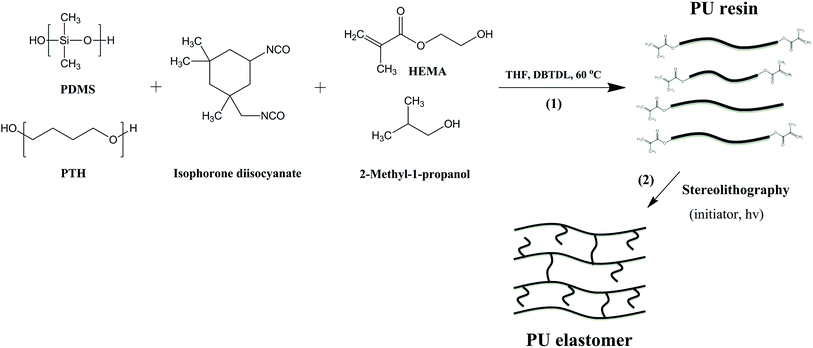 | ||
| Scheme 1 Synthesis of photo-curable polyurethane resin (1) and formation of polyurethane elastomer via stereolithography (2). | ||
The average number molecular weight (Mn) and molar-mass dispersity (Đ) of synthesized polymer were determined using a gel permeation chromatography (GPC) to be 3600 g mol−1 and 3.27, respectively (Fig. S1, ESI†). Low Mn and wide Đ of resin provide its low viscosity, in which the low molecular weight chains can play as diluents. The chemical structure of resin was confirmed by 1H-NMR spectra exhibiting the characteristic peaks of all components and the peak of methacrylate protons at 5.55 and 6.05 ppm (Fig. S2, ESI†).
For stereolithographic fabrication, the synthesized resin was mixed with 0.1 wt% of Irgacure® TPO-L (ethyl-2,4,6-trimethylbenzoyl phosphine) as initiator and 0.1 wt% of Orasol Orange G as dye (both relative to the amount of resin). The mixing step was carried out for 24 h to ensure the uniformity of the mixture. Subsequently, stereolithography fabrication of resin was performed upon illumination with blue light of a wavelength of 400–500 nm, with a peak at 440 nm (Fig. 1a).
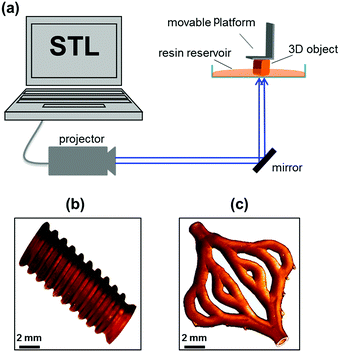 | ||
| Fig. 1 Schematic presentation of stereolithography fabrication (a). Photographs of printed tube (b) and hollow structure artificial skin blood vessel model (c) from polyurethane resin. | ||
The printing ability of resin was evaluated by two factors: viscosity and curing rate. The viscosity of the resin was investigated on Physica MCR 301 rheometer (Anton Paar) using cylinder cup geology CC27 at a constant shear stress of 0.5 MPa. The resin showed viscosity of 2200 mPa s at room temperature, which is acceptable for our stereolithography apparatus (Fig. S3, ESI†). The curing of resin was tested on the projector of the stereolithography system. The resin was casted on a glass slide and illuminated with blue light with wavelength of 400–500 nm. The curing time of 10 seconds was sufficient for 30 μm layer thickness.
Firstly, we demonstrated printing ability of synthesized resin with tubular model. Then, we carried out printing of the resin with artificial skin blood vessel model, which has highly complex architecture. The artificial skin blood vessel contains various tube diameters and wall thicknesses, among which the thinnest wall is 200 μm and smallest tube diameter is 1 mm. The printing process of the resin worked well with a curing time of 10 seconds for one layer of 30 μm in thickness. After printing, the samples were washed thoroughly with ethanol to remove uncured resin. The printed samples exhibited high accuracy and precision as designed models (Fig. 1b and c).
The photo-curable elastomer exhibited high toughness and good mechanical strength. In particular, Young's modulus, tensile strength and elongation at break of elastomer were measured to be 2.5 MPa, 3.7 MPa, and 195%, respectively, by tensile testing (Fig. S4, ESI†). The results are comparable and may have advantages over reported photo-curable elastomers for soft tissue engineering applications.17–19
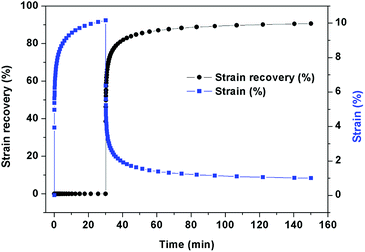
The elasticity of elastomer was investigated with static creep recovery test on a dynamic mechanical analysis system (DMA, Q800, TA Instrument). The samples were applied with a constant stress of 0.25 MPa for 30 min at 30 °C. Then, the force was released to let the samples recover back to the original shape. The strain recovery (%) was recorded along with recovery time up to 120 min. The plot of strain recovery of elastomer along with time is shown in Fig. 2. The result showed that elastomer showed a good elastic recovery behaviour, which recovered to 60% within 30 seconds after releasing force. The maximum strain recovery reached more than 90%.
The elastomer showed a broaden glass transition temperature (Tg) in range from −77.5 °C to −71.8 °C (Fig. S5, ESI†). This could be due to complexity in arrangement of monomers and different polymer chain lengths of resin as confirmed by the PDI value. Moreover, broaden Tg of elastomer was also observed in DMA results (Fig. S6, ESI†). Two broad peaks of Tg were attributed to the different rigidity and chain length, the complexity of segments, and the variety of crosslink density of the resin.
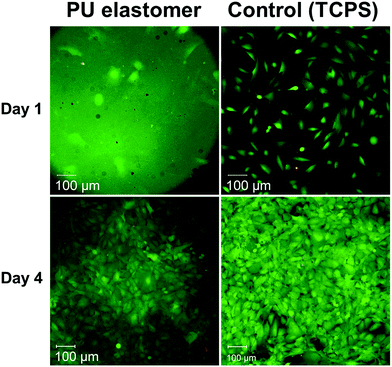
The in vitro cytocompatibility of developed elastomer was evaluated by cytotoxicity testing. The polyurethane elastomer substrates were cultured with the 3T3 mouse fibroblast cell line, which is commonly used to assess cytotoxicity of potential substrates for cell growth. Fig. 3 shows fluorescence microscopy images of fluorescein diacetate/GelRed™-stained 3T3 cells growing on PU elastomer and tissue culture polystyrene (TCPS) control substrates after 1 and 4 days of cell culture. Unfortunately, the PU elastomer caused a high background fluorescence allowing visualization of the cells only after short exposition time and extreme contrast adjustment of the raw data. The situation was clearly better at 4 days, when the cell layer covered the material surface. Nevertheless, the increase of the cell number on the samples was unambiguous, so that exact counting from multiple images was not necessary. Fig. 3 illustrates cell densities were rising from about 2000 cells per cm2 at day 1 (artificially low, obviously by the shape of the specimen) to about 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 at day 4 (in comparison to the reference, where cell numbers of about 12
000 cells per cm2 at day 4 (in comparison to the reference, where cell numbers of about 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 at day 1 and about 77
500 at day 1 and about 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 at day 4 have been estimated). The percentage of dead cells was in general below 1% at day 1 and amounted to 2.9% at the elastomer and to 2.8% at the reference at day 4. The elastomer substrate showed excellent cell adhesion and proliferation of 3T3 cells, suggesting that the elastomer is a cytocompatible material and could be applied in biomedical applications.
500 at day 4 have been estimated). The percentage of dead cells was in general below 1% at day 1 and amounted to 2.9% at the elastomer and to 2.8% at the reference at day 4. The elastomer substrate showed excellent cell adhesion and proliferation of 3T3 cells, suggesting that the elastomer is a cytocompatible material and could be applied in biomedical applications.
Conclusions
In summary, we have demonstrated herein a novel photo-curable polyurethane. The resin was applied in stereolithography without need of any diluent. We also demonstrated successfully printing of artificial blood vessel with high accuracy and precision. The printed elastomer showed good mechanical strength and elasticity. More important, the resulting elastomer exhibited excellent cell adhesion and proliferation in cytotoxicity tests with cell viabilities higher than 97%. The resin can be considered a promising candidate for the fabrication of permanent elastomeric biomedical devices by stereolithographic technique.Acknowledgements
We gratefully acknowledge the financial support from the European Commission for funding ArtiVasc 3D-project (FP7, grant agreement No. 263416) and Academy of Finland Funding (No. 12137759 and 13272725).Notes and references
- V. P. Shastri, Curr. Pharm. Biotechnol., 2003, 4, 331–337 CAS
.
- P. Roach, D. Eglin, K. Rohde and C. C. Perry, J. Mater. Sci.: Mater. Med., 2007, 18, 1263–1277 CrossRef CAS PubMed
.
- B. D. Ratner and S. J. Bryant, Annu. Rev. Biomed. Eng., 2004, 6, 41–75 CrossRef CAS PubMed
.
- B. Wendel, D. Rietzel, F. Kühnlein, R. Feulner, G. Hülder and E. Schmachtenberg, Macromol. Mater. Eng., 2008, 293, 799–809 CrossRef CAS
.
- M. E. Hoque, Y. L. Chuan and I. Pashby, Biopolymers, 2012, 97, 83–93 CrossRef CAS PubMed
.
- A. D. Lantada and P. L. Morgado, Annu. Rev. Biomed. Eng., 2012, 14, 73–96 CrossRef CAS PubMed
.
- S. M. Peltola, F. P. W. Melchels, D. W. Grijpma and M. Kellomäki, Ann. Med., 2008, 40, 268–280 CrossRef CAS PubMed
.
- Y.-J. Seol, T.-Y. Kang and D.-W. Cho, Soft Matter, 2012, 8, 1730–1735 RSC
.
- R. A. Watson, J. Surg. Educ., 2014, 71, 14–17 CrossRef PubMed
.
- M. W. Naing, C. K. Chua, K. F. Leong and Y. Wang, Rapid Prototyping J., 2005, 11, 249–259 CrossRef
.
-
S. Corbel, O. Dufaud and T. Roques-Carmes, Materials for Stereolithography, Springer, USA, 2011, ch. 6, pp. 141–159 Search PubMed
.
- F. P. W. Melchels, J. Feijen and D. W. Grijpma, Biomaterials, 2010, 31, 6121–6130 CrossRef CAS PubMed
.
- F. P. W. Melchels, J. Feijen and D. W. Grijpma, Biomaterials, 2009, 30, 3801–3809 CrossRef CAS PubMed
.
- S. Schüller-Ravoo, J. Feijen and D. W. Grijpma, Macromol. Biosci., 2011, 11, 1662–1671 CrossRef PubMed
.
-
N. D. Luong, L. H. Sinh and J. Seppälä, US Pat., 62/013174, 2014
.
-
A. Burke and N. Hasirci, Polyurethane in Biomedical Applications, Springer, USA, 2004, vol. 553, ch. 7, pp. 83–101 Search PubMed
.
- B. G. Amsden, G. Misra, F. Gu and H. M. Younes, Biomacromolecules, 2004, 5, 2479–2486 CrossRef CAS PubMed
.
- C. L. E. Nijst, J. P. Bruggeman, J. M. Karp, L. Ferreira, A. Zumbuehl, C. J. Bettinger and R. Langer, Biomacromolecules, 2007, 8, 3067–3073 CrossRef CAS PubMed
.
- E. Bat, B. H. M. Kothman, G. A. Higuera, C. A. van Blitterswijk, J. Feijen and D. W. Grijpma, Biomaterials, 2010, 31, 8696–8705 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section: materials, synthesis, stereolithography fabrication, characterizations (GPC, 1H-NMR, rheology, tensile, DSC, and DMA). See DOI: 10.1039/c6ra05045j |
| ‡ These authors contributed equally in this work. |
| This journal is © The Royal Society of Chemistry 2016 |