G-quadruplex–hemin DNAzyme molecular beacon probe for the detection of methamphetamine
Abstract
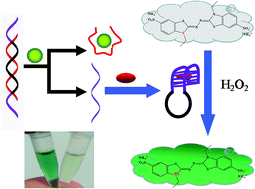
In this work, a simple, cost-effective, and label-free biosensor was constructed for methamphetamine (METH) detection. The biosensor consists of a G-quadruplex–hemin DNAzyme molecular beacon (DNAzyme MB), a METH aptamer, and a colorimetric substrate. The DNAzyme MB loses peroxidase activity when it hybridizes with the METH aptamer. In the presence of METH, DNAzyme MB dissociates from the inactive hybrid due to preferable hybridization of METH with the aptamer. This process recovers the activity of DNAzyme MB, which catalyzes a reaction with the colorimetric substrate to yield measurable signals. Under optimized conditions, a detection limit as low as 0.5 nM (74.6 ng L−1) was achieved. Common illicit drugs were found to have little interference on detection of METH. Recoveries of METH spiked in urines of addicts were greater than 85%. Good agreement was observed between METH concentrations in urines determined by the sensor and by liquid chromatography-tandem mass spectrometer. These results indicate that the G-quadruplex–hemin DNAzyme MB probe holds promise to detect METH not only in biological samples, but also in environmental matrices.

 Please wait while we load your content...
Please wait while we load your content...