Preparation and preliminary application of 5-HMF@SiO2 micro-particles†
Cheng Wang‡
,
Huaizhen He‡,
Yuanyuan Lin,
Limin Huang,
Meng Sun,
Tao Zhang and
Langchong He*
School of Medicine, Xi'an Jiaotong University, Xi'an 710061, P. R. China. E-mail: helc@mai.xjtu.edu.cn; Fax: +86-29-82655451; Tel: +86-29-82655451
First published on 5th July 2016
Abstract
A new approach for the surficial-modification of silica with 5-HMF as a functional molecule was first designed. The infrared spectrum, X-ray photoelectron spectroscopy and elemental analysis showed that the modified-SiO2 particles were successfully established. Full dispersion of SiO2 and the introduction of gallic acid were confirmed to be favorable for the 5-HMF content. The retention behavior of bovine serum albumin on the modified-SiO2 particle suggested that the novel particles could serve as a promising stationary phase on the identification of the particular proteins.
1. Introduction
5-Hydroxymethylfurfural (5-HMF) is a furan-based compound derived from the dehydration of fructose or glucose, present in coffee, baking products and intravenous fluids.1 5-HMF can be utilized as an intermediate for valuable chemicals and fuels instead of fossil sources such as 2,5-dihydroxymethylfuran, 2,5-bis (hydroxymethyl) tetrahydrofuran, 2,5-diymethylfuran and 2,5-furandicarboxylic acid.2 Consequently, many studies have focused on the conversion of 5-HMF by fructose or glucose. Metal catalysts, ionic liquids and two-phase system have achieved significant progress on high selectivity and efficient conversion.3 5-HMF has also been revealed to possess various pharmacological activities, for instance, anti-oxidant and anti-proliferative activity, modifying intracellular sickle haemoglobin, inhibiting sickling of red blood cells, protection of acute hypobaric hypoxia and enhance cognitive function.4 5-HMF can be detected in processed Fructus Corni, a Chinese medicine used to invigorate the liver and kidney; it was also presented in processed steamed ‘Rehmanniae Radix’ for several diseases such as anemia and diabetes.5 Although some research found the human colon cancer cell line CaCo-2, the human epithelial kidney cell line HEK 293, and the mouse lymphoma cell line L5178Y showed DNA damage in the presence of 5-HMF, the contradictory results maybe caused by 5-sulfooxymethylfurfural, which is a more harmful metabolite of 5-HMF.6 Therefore, more study of 5-HMF is needed.Inorganic/organic hybrid particles based on the surface modification of inorganic fillers have attracted increasing attention because of their combination of inorganic materials and organic molecules, and have been applied in numerous aspects of catalysis, materials, pharmaceuticals, electrochemical sensors and chromatography.7 As an important part of inorganic particles, silica has attracted considerable interest for its significant properties such as excellent mechanical properties, high specific surface area, facile synthesis and commercial availability.8 In addition, the presence of silanol groups on the surface of silica gel is in favor of chemical modification.9 Silane coupling agents have been used on a large scale for the surface modification of silica gel for their unique structural function. Silicon ether groups can react with silica gel under moderate conditions, and the terminal groups (including vinyl, epoxy, methacryl, amino, mercapto, and chlorine) can react with many types of organic compounds. In that way, inorganic silica has some organic functions. At present, great efforts have been placed toward fabrication to expand the properties and applications of silica-based composites by assembling small organic molecules on the surface,10 many of them demonstrated excellent separation as stationary phases in liquid chromatography.11
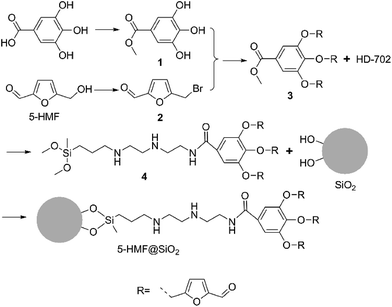
Although many types of hybrid particles have served in various areas, there has been little study on protein recognition. Herein, we describe an appropriate strategy for the fabrication of 5-HMF@SiO2 microspheres, and attempt to expand its applications to protein recognition. As illustrated in Scheme 1, 5-HMF@SiO2 micro-particles were prepared through five simple and convenient reactions. Gallic acid was introduced to enhance the density of 5-HMF using its three phenolic hydroxyl groups reacting with 5-HMF bromide. N-(2-Aminoethyl)-N′-[3-(dimethoxymethylsilyl)propyl]-1,2-ethanediamine (HD-702), a silane coupling agent, was used as a linker for SiO2 and the gallic acid derivative contained 5-HMF, which also served to decrease the impact of SiO2 to 5-HMF by its ten-atom long-chain. First, compounds 1 and 2 were synthesized by concise esterification and bromination. Intermediate 3 was obtained by the etherification of compound 1 with 2. The aminolysis of intermediate 3 with N-(2-aminoethyl)-N′-[3-(dimethoxymethylsilyl)propyl]-1,2-ethanediamine gained the critical organosilylated ligand 4. The target 5-HMF@SiO2 micro-particle was finally prepared by hydrolysis with 7 μm silica gel. The target particle was determined by infrared spectroscopy (IR), elemental analysis (EA), X-ray photoelectron spectroscopy (XPS) and nitrogen adsorption and desorption analysis. The synthesized particle was used as a novel stationary phase in liquid chromatography for preliminary protein recognition of bovine serum albumin.
2. Experimental sections
2.1. Materials
All reagents and solvents were purified and dried using standard techniques. The melting points were determined on an X-4 apparatus without correction. The infrared (IR) spectra were obtained on a Shimadzu FT-IR 440 spectrometer in the 4000–500 cm−1 range. The mass spectra were performed on a Shimadzu GC-MSQP 2010 instrument (Shimadzu, Japan). 1H NMR and spectra were obtained on a Bruker AVANCF 400 MHz instrument with TMS as the internal standard. The chemical shift multiplicities were reported as follows: s, singlet; d, doublet; t, triplet; m, multiplet. XPS was carried out on a Kratos AXIS ULtrabld energy dispersive X-ray spectrometer (Manchester, England). Elements analysis was measured on vario MACRO cube Elementar (Hanau, Germany). High-resolution mass spectra were obtained on a Shimadzu HPLC-IT-TOF-MS (Shimadzu, Japan).2.2. The preparation of the samples
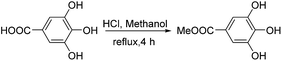
To a solution of gallic acid (3.00 g, 17.6 mmol) in methanol was added concentrated hydrochloric acid (6 mL). The solvent was evaporated after reflux for 4 h and the residue was placed in a vacuum overnight to afford the pure product (2.61 g, 80.0% yield). White solid; mp: 200–201 °C; ESI-MS (m/z): 183.0413 [M]+. 1H NMR (400 MHz, CD3OD): δ 7.04 (s, 2H, –Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 3.82 (s, 3H, –OC
), 3.82 (s, 3H, –OC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3).
3).
Trimethylbromosilane was added to a solution of 5-hydroxymethylfurfural (1.26 g, 10.0 mmol). The resulting solution was stirred at r.t. for another 4 h. The reaction was terminated using a saturated NaHCO3 solution. The organic phase was washed with water (1 × 100 mL) and brine solution (2 × 50 mL), dried over anhydrous Na2SO4, and evaporated to obtained 5-(bromomethyl) furan-2-carbaldehyde (1.46 g, 77.2% yield). Brown solid; EI-MS (m/z): 187.9 [M]+. 1H NMR (400 MHz, CDCl3): δ 9.62 (s, 1H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) O), 7.20 (d, J = 3.6 Hz, 1H, –CH
O), 7.20 (d, J = 3.6 Hz, 1H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CCHO), 6.60 (d, J = 3.6 Hz, 1H, –C
CCHO), 6.60 (d, J = 3.6 Hz, 1H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCCHO), 4.50 (s, 2H, –C
CHCCHO), 4.50 (s, 2H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2Br).
2Br).
To a solution of gallicin (0.41 g, 2.2 mmol) in DMF was added anhydrous K2CO3 (1.52 g, 11.0 mmol). After stirring for 0.5 h, KI (1.28 g, 7.7 mmol) and compound 2 (1.46 g, 7.7 mmol) were added to the solution. The mixture was filtered after heating at 80 °C for 15 h. The solution was diluted with water and extracted by ethyl acetate. The organic phase was washed with brine solution (2 × 50 mL), dried over anhydrous Na2SO4, and evaporated. The residue was purified by column chromatography (petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 2
ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Orange solid; ESI-MS (m/z): 507.0955 [M]+. 1H NMR (400 MHz, CDCl3) δ 9.67 (s, 2H, –CHO), 9.59 (s, 1H, –CHO), 7.43 (s, 2H, –ArH), 7.27 (d, J = 3.6 Hz, 2H, –CH
3). Orange solid; ESI-MS (m/z): 507.0955 [M]+. 1H NMR (400 MHz, CDCl3) δ 9.67 (s, 2H, –CHO), 9.59 (s, 1H, –CHO), 7.43 (s, 2H, –ArH), 7.27 (d, J = 3.6 Hz, 2H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CCHO), 7.18 (d, J = 3.5 Hz, 1H, –CH
CCHO), 7.18 (d, J = 3.5 Hz, 1H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CCHO), 6.69 (d, J = 3.6 Hz, 2H, –C
CCHO), 6.69 (d, J = 3.6 Hz, 2H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCCHO), 6.58 (d, J = 3.5 Hz, 1H, –C
CHCCHO), 6.58 (d, J = 3.5 Hz, 1H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCCHO), 5.18 (s, 4H, –C
CHCCHO), 5.18 (s, 4H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2Br), 5.17 (s, 2H, –C
2Br), 5.17 (s, 2H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2Br), 3.94 (s, 3H, –OC
2Br), 3.94 (s, 3H, –OC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3).
3).
HATU (0.68 g, 1.8 mmol) was added to a mixture of compound 3 (0.51 g, 1.0 mmol) and HD 702 (0.25 g, 1.0 mmol) in THF; the solution was stirred at r.t. for 6 h. The solvent was evaporated and washed with dried methanol and DMSO to obtain the product. 1H NMR (400 MHz, DMSO-d6): δ 8.33 (s, 1H), 6.88 (s, 2H), 6.66 (s, 1H), 3.51 (s, 3H), 2.51 (s, 10H), 1.36 (s, 2H), 0.86 (s, 1H).
2.2.6.1 General procedure A for 5-HMF@SiO2-1. To a solution of compound 4 (0.5 g) in ethanol (50 ml) was added silica gel (2.0 g, 7 μm). The mixture was heated at 70 °C for 8 h; filtered; successively washed with dimethyl sulfoxide, tri-distilled water and methanol; and dried at 60 °C.
2.2.6.2 General procedure B for 5-HMF@SiO2-2. To a solution of compound 4 (0.5 g) in toluene (500 ml) was added silica gel (2.0 g, 7 μm), the mixture was heated under reflux for 8 h; filtered; successively washed with dimethyl sulfoxide, tri-distilled water and methanol; and dried at 60 °C.
2.3. Column packing
5-HMF@SiO2-2 micro-particle was homogenised with tri-distilled water and packed into stainless steel columns (50 mm × 4.6 mm) as the stationary phase. For comparison, the SiO2 were also pumped into the columns with the same dimensions.2.4. Procedure of chromatography
A 2.5 × 10−4 TFA was used as the mobile phase in the chromatography experiments to investigate the retention behaviour of BSA. Chromatography was performed at a detection wavelength of 284 nm with a flow rate of 0.2 mL min−1 and 1.0 mL min−1.3. Results and discussion
3.1. Preparation of 5-HMF@SiO2
The synthetic route is shown in Scheme 1. Gallic acid was catalyzed by hydrochloric acid under reflux in methanol to afford gallicin (1). The bromination of 5-hydroxymethylfurfural by trimethylbromosilane obtained compound 2. The high resolution mass spectrum (Fig. S5–S7†) revealed mono-substituted (m/z 291.0516), bi-substituted (m/z 399.0741) and tri-substituted (m/z 507.0955) products of gallicin. A great excess compound 2 was beneficial to form the tri-substituted product of gallicin, finally, improving the concentration of 5-hydroxymethylfurfural in 5-HMF@SiO2. 5-HMF@SiO2 was obtained by two different conditions, ethanol exactly dissolved 4 and a great excess volume of toluene served as the solvents. The reactions were performed at 70 °C and reflux temperature of toluene. 5-HMF@SiO2-1 and 5-HMF@SiO2-2 were obtained separately using the abovementioned two methods. To remove the unreacted components, surface-modified SiO2 particles were washed successively with dimethylsulfoxide, tri-distilled water and methanol.3.2. Characterization of 5-HMF@SiO2
A comparison of the IR spectra between original and surface-modified SiO2 shown in Fig. 1 confirmed the relationship and distinction between SiO2 and 5-HMF@SiO2. In the spectrum of the original SiO2 particles, the characteristic absorption peak at 3447 cm−1 in the spectrum was assigned to the –OH group. The vibration band of 1101 cm−1 belonged to the Si–O asymmetric stretching band. Moreover, the vibration peaks at 467 and 797 cm−1 were attributed to the Si–O bond rocking and bending, respectively. The characteristic peaks of 5-HMF@SiO2 at 2929 and 2856 cm−1 were assigned to the C–H asymmetric and symmetric stretching vibration of –CH2. The stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in 5-HMF appeared at about 1650 cm−1. These new peaks revealed the successful grafting of SiO2 particles.12
O in 5-HMF appeared at about 1650 cm−1. These new peaks revealed the successful grafting of SiO2 particles.12
Furthermore, XPS was performed to investigate the elements, functional groups and the difference between the original and surface-modified SiO2. Fig. 2 showed that original silica gel particle exhibited Si (Si 2s and Si 2p) and O peaks and a C signal; the last peak was assigned to adventitious contamination. The silicon signals were the result of the silicon oxide layer and the underlying silicon substrate. Moreover, the O 1s peak at about 530 eV was also assigned to silicon oxide. The both processed samples showed C, O, and Si signals, but they also showed a N 1s peak at about 400 eV.
The weak peak of N 1s at 397 eV in 5-HMF@SiO2-1 and 5-HMF@SiO2-2 indicated that surface-modified SiO2 contained N compared to the original SiO2. The N 1s and C 1s core-level spectrum in Fig. 3a–d further revealed the difference between the three particles. The C 1s spectrum was curve-fitted with four peaks at about 284.6, 285.6, 288.1 and 290.0 eV, which were attributed to C–C/C–H, C–O, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and π–π* species, respectively. The N 1s spectrum was resolved into two peaks, major 398.4 and minor 400.1 eV. These suggested that the surface modification of SiO2 was successful. In addition, the XPS results in Fig. 3c also showed that the nitrogen content in 5-HMF@SiO2-2 was apparently higher than that of 5-HMF@SiO2-1. This meant that the great excess volume and reflux temperature of toluene in procedure B benefited the content of 5-HMF in 5-HMF@SiO2.
C, and π–π* species, respectively. The N 1s spectrum was resolved into two peaks, major 398.4 and minor 400.1 eV. These suggested that the surface modification of SiO2 was successful. In addition, the XPS results in Fig. 3c also showed that the nitrogen content in 5-HMF@SiO2-2 was apparently higher than that of 5-HMF@SiO2-1. This meant that the great excess volume and reflux temperature of toluene in procedure B benefited the content of 5-HMF in 5-HMF@SiO2.
The comparison of elemental composition for SiO2, 5-HMF@SiO2-1 and 5-HMF@SiO2-2 was shown in Table 1. The nitrogen and carbon contents in 5-HMF@SiO2 were higher than that of SiO2 after surface-modification. In addition, a comparison between 5-HMF@SiO2-1 and 5-HMF@SiO2-2 also suggested that the fully-dispersed SiO2 of procedure B was conducive to the 5-HMF content in 5-HMF@SiO2. However, the low-proportion of nitrogen directed to a low amount of linker on the surface of SiO2, highlighting the need for further study to increase the content of the modified compounds.
| Sample | Element | ||||
|---|---|---|---|---|---|
| N [%] | C [%] | H [%] | C/N | C/H | |
| SiO2 | 0.13 | 0.35 | 0.216 | 2.7164 | 1.6355 |
| 5-HMF@SiO2-1 | 0.24 | 1.15 | 0.355 | 5.7916 | 3.2394 |
| 5-HMF@SiO2-2 | 0.47 | 3.22 | 0.696 | 6.7979 | 4.6230 |
The microscopic pore structure was measured by nitrogen adsorption and desorption analysis. The parameters were shown in Table 2. The BET surface area (SBET), Langmuir surface area (SLangmuir) and total pore volume (Vp) of modified silica gel were all lower than those of original silica, indicating that the attachments were packed in the mesopores or adhered to the surface of the silica gels. A comparison between the two grafted particles also showed that procedure B was more suitable for surface-modification than procedure A.
| Sample | SBET (m2 g−1) | SLangmuir (m2 g−1) | Vp (mL g−1) |
|---|---|---|---|
| SiO2 | 220.00 | 326.12 | 0.54 |
| 5-HMF@SiO2-1 | 74.32 | 108.28 | 0.43 |
| 5-HMF@SiO2-2 | 34.98 | 51.03 | 0.38 |
3.3. Retention behaviour
Bovine serum albumin was reported to conjugate with 5-HMF.13 In this section, the more sufficiently modified silica-based particles, 5-HMF@SiO2-2, was operated as a novel stationary phase to investigate its chromatographic property for the retention behaviour of bovine serum albumin. The retention time of bovine serum albumin on chromatographic columns with 5-HMF@SiO2-2 as the stationary phase was apparently prolonged compared to that of the SiO2 stationary phase, which were 2.02 min for 5-HMF@SiO2-2 and 0.29 min for SiO2 at a rate of 0.2 ml min−1 (Fig. 4a). Their retention times were separately 0.42 min and 0.07 min at a rate of 1.0 ml min−1. The capacity of acid-resisting of the new stationary phase was tested over a wide range of acidity, 1 × 10−6 to 1 × 10−2 M of TFA. In addition, the column with the modified stationary phase exhibited good reproducibility after serving for two weeks. | ||
| Fig. 4 Retention behaviour of bovine serum albumin on SiO2 and 5-HMF@SiO2-2 ((a) 0.2 ml min−1; (b) 1.0 ml min−1). | ||
4. Conclusions
In summary, we succeeded in the design of a novel stationary phase by the surface-modification of silica gel. 5-HMF was immobilized as a functional molecule on the surface of silica gel. Gallic acid was applied to increase the content of 5-HMF in 5-HMF@SiO2. The particle was synthesized successfully but at an unsatisfactory bond rate. The retention time of bovine serum albumin on 5-HMF@SiO2 was remarkably prolonged compared to SiO2. Because 5-HMF exhibits a range of pharmacological activities, the retention behaviour of bovine serum albumin on the novel stationary phase showed that the prepared particles could be used as a promising new-type stationary phase for the separation and recognition of proteins analysed with a capacity of coupling with 5-HMF. Further study in this area is currently underway.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81230079, 81202494).References
- (a) A. Ramírez-Jiménez, B. García-Villanova and E. Guerra-Hernandez, Food Res. Int., 2000, 33, 833 CrossRef; (b) J. A. Rufián-Henares, C. Delgado-Andrade and F. J. Morales, J. Cereal Sci., 2006, 43, 63 CrossRef; (c) R. L. Prior, X. Wu and L. Gu, J. Agric. Food Chem., 2006, 54, 3744 CrossRef CAS PubMed; (d) T. Husoy, M. Haugen, M. Murkovic, D. Jobstl, L. H. Stolen, T. Bjellaas, C. Ronningborg, H. Glatt and J. Alexander, Food Chem. Toxicol., 2008, 46, 3697 CrossRef CAS PubMed; (e) M. Ciulu, I. Floris, V. M. Nurchi, A. Panzanelli, M. I. Pilo, N. Spano and G. Sanna, J. Agric. Food Chem., 2015, 63, 4190 CrossRef CAS PubMed; (f) B. E. Demirhan, B. Demirhan, C. Sönmez, H. Torul, U. Tamer and G. Yentür, J. Dairy Sci., 2015, 98, 818 CrossRef PubMed.
- (a) S. P. Simeonov, J. A. S. Coelho and C. A. M. Afonso, ChemSusChem, 2012, 5, 1388 CrossRef CAS PubMed; (b) T. Stahlberg, W. J. Fu, J. M. Woodley and A. Riisager, ChemSusChem, 2011, 4, 451 CrossRef CAS PubMed; (c) S. Subbiah, S. P. Simeonov, J. M. Esperança, L. P. N. Rebelo and C. A. Afonso, Green Chem., 2013, 15, 2849 RSC; (d) A. D. Sutton, F. D. Waldie, R. Wu, M. Schlaf, A. Louis III and J. C. Gordon, Nat. Chem., 2013, 5, 428 CrossRef CAS PubMed; (e) S. Dutta, S. De and B. Saha, ChemPlusChem, 2012, 77, 259 CrossRef CAS.
- (a) Y. Román-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982 CrossRef PubMed; (b) Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Nature, 2006, 312, 1933 Search PubMed; (c) H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597 CrossRef CAS PubMed; (d) M. R. Grochowski, W. Yang and A. Sen, Chem.–Eur. J., 2012, 18, 12363 CrossRef CAS PubMed; (e) C. Loerbroks, J. van Rijn, M.-P. Ruby, Q. Tong, F. Schüth and W. Thiel, Chem.–Eur. J., 2014, 20, 12298 CrossRef CAS PubMed; (f) Y. Zhang, E. A. Pidko and E. J. M. Hensen, Chem.–Eur. J., 2011, 17, 5281 CrossRef CAS PubMed; (g) Q. Cao, X. Guo, J. Guan, X. Mu and D. Zhang, Appl. Catal., A, 2011, 403, 98 CrossRef CAS; (h) C. V. McNeff, D. T. Nowlan, L. C. McNeff, B. Yan and R. L. Fedie, Appl. Catal., A, 2010, 384, 65 CrossRef CAS; (i) X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr, Catal. Commun., 2009, 10, 1771 CrossRef CAS.
- (a) D. D. Kitts, X. M. Chen and H. Jing, J. Agric. Food Chem., 2012, 60, 6718 CrossRef CAS PubMed; (b) B. Sachse, W. Meinl, Y. Sommer, H. Glatt, A. Seidel and B. H. Monien, Arch. Toxicol., 2014, 1 Search PubMed; (c) O. Abdulmalik, M. K. Safo, Q. Chen, J. Yang, C. Brugnara, K. Ohene-Frempong, D. J. Abraham and T. Asakura, Br. J. Haematol., 2005, 128, 552 CrossRef CAS PubMed; (d) A. Hannemann, U. M. Cytlak, D. C. Rees, S. Tewari and J. S. Gibson, J. Physiol., 2014, 592, 4039 CrossRef CAS PubMed; (e) M. M. Li, L. Y. Wu, T. Zhao, K. W. Wu, L. Xiong, L. L. Zhu and M. Fan, Cell Stress Chaperones, 2011, 16, 529 CrossRef CAS PubMed; (f) Y. Lee, Q. Gao, E. Kim, Y. Lee, S. J. Park, H. E. Lee, D. S. Jang and J. H. Ryu, Pharmacol., Biochem. Behav., 2015, 134, 57 CrossRef CAS PubMed.
- (a) L. J. K. Durling, L. Busk and B. E. Hellman, Food Chem. Toxicol., 2009, 47, 880 CrossRef CAS PubMed; (b) A. S. Lin, K. Qian, Y. Usami, L. Lin, H. Itokawa, C. Hsu, S. C. Morris-Natschke and K. H. Lee, J. Nat. Med., 2007, 62, 164 CrossRef PubMed.
- (a) L. J. K. Durling, L. Busk and B. E. Hellman, Food Chem. Toxicol., 2009, 47, 880 CrossRef CAS PubMed; (b) I. Severin, C. Dumont, A. Jondeau-Cabaton, V. Graillot and M. C. Chagnon, Toxicol. Lett., 2010, 192, 189 CrossRef CAS PubMed; (c) C. Janzowski, V. Glaab, E. Samimi, J. Schlatter and G. Eisenbrand, Food Chem. Toxicol., 2000, 38, 801 CrossRef CAS PubMed.
- (a) Y. C. Lee, M. Shlyankevich, H. K. Jeong, J. S. Douglas and Y. J. Surh, Biochem. Biophys. Res. Commun., 1995, 209, 996 CrossRef CAS PubMed; (b) B. H. Monien, H. Frank, A. Seidel and H. Glatt, Chem. Res. Toxicol., 2009, 22, 1123 CrossRef CAS PubMed; (c) H. Glatt, H. Schneider and Y. Liu, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2005, 580, 41 CrossRef CAS PubMed.
- (a) F. Carniato, L. Tei, A. Arrais, L. Marchese and M. Botta, Chem.–Eur. J., 2013, 19, 1421 CrossRef CAS PubMed; (b) W. Chaikittisilp, A. Sugawara, A. Shimojima and T. Okubo, Chem.–Eur. J., 2010, 16, 6006 CrossRef CAS PubMed; (c) A. T. Dickschat, F. Behrends, M. Bühner, J. Ren, M. Wei, H. Eckert and A. Studer, Chem.–Eur. J., 2012, 18, 16689 CrossRef CAS PubMed; (d) J. Djamil, S. A. W. Segler, W. Bensch, U. Schürmann, M. Deng, L. Kienle, S. Hansen, T. Beweries, L. von Wüllen, S. Rosenfeldt, S. Förster and H. Reinsch, Chem.–Eur. J., 2015, 21, 8918 CrossRef CAS PubMed; (e) N. Mizoshita, Y. Goto, M. P. Kapoor, T. Shimada, T. Tani and S. Inagaki, Chem.–Eur. J., 2009, 15, 219 CrossRef CAS PubMed; (f) R. Sauer, P. Froimowicz, K. Schöller, J.-M. Cramer, S. Ritz, V. Mailänder and K. Landfester, Chem.–Eur. J., 2012, 18, 5201 CrossRef CAS PubMed; (g) M. Antoniett, M. Breulmann, C. G. Göltner, H. Cölfen, K. K. Wong, D. Walsh and S. Mann, Chem.–Eur. J., 1998, 4, 2493 Search PubMed.
- (a) M. Abdollahi, M. Rouhani, M. Hemmati and P. Salarizadeh, Polym. Int., 2013, 62, 713 CrossRef CAS; (b) F. Niepceron, B. Lafitte, H. Galiano, J. Bigarré, E. Nicol and J. F. Tassin, J. Membr. Sci., 2009, 338, 100 CrossRef CAS; (c) B. Radhakrishnan, R. Ranjan and W. J. Brittain, Soft Matter, 2006, 2, 386 RSC; (d) J. A. Libera, J. W. Elam and M. J. Pellin, Thin Solid Films, 2008, 516, 6158 CrossRef CAS; (e) O. Prucker, J. Rühe, Macromolecules 1998, 31, 592 Search PubMed; (f) C. J. Brinker, R. K. Brow, D. R. Tallant and R. J. Kirkpatrick, J. Non-Cryst. Solids, 1990, 120, 26 CrossRef CAS.
- (a) J. G. Croissant, X. Cattoën, M. Wong Chi Man, J. O. Durand and N. M. Khashab, Nanoscale, 2015, 7, 20318 RSC; (b) P. M. A. Machado, L. M. Lube, M. D. E. Tiradentes, C. Fernandes, C. A. Gomes, A. M. Stumbo, R. A. S. San Gil, L. C. Visentin, D. R. Sanchez, V. L. A. Frescura, J. S. A. Silva and A. Horn, Appl. Catal., A, 2015, 507, 119 CrossRef CAS; (c) M. Opanasenko, P. Štěpnička and J. Čejka, RSC Adv, 2014, 4, 65137 RSC; (d) V. Sacchetto, C. Bisio, D. F. Olivas Olivera, G. Paul, G. Gatti, I. Braschi, G. Berlier, M. Cossi and L. Marchese, J. Phys. Chem. C, 2015, 119, 24875 CrossRef CAS; (e) F. Vibert, S. R. A. Marque, E. Bloch, S. Queyroy, M. P. Bertrand, S. Gastaldi and E. Besson, Chem. Sci., 2014, 5, 4716 RSC; (f) X. L. Zhang, H. Yamada, T. Saito, T. Kai, K. Murakami, M. Nakashima, J. Ohshita, K. Akamatsu and S. I. Nakao, J. Membr. Sci., 2016, 499, 28 CrossRef CAS.
- (a) T. Liang, Q. Fu, A. Shen, H. Wang, Y. Jin, H. Xin, Y. Ke, Z. Guo and X. Liang, J. Chromatogr. A, 2015, 1388, 110 CrossRef CAS PubMed; (b) X. Liu, J. Feng, X. Sun, Y. Li and G. Duan, Anal. Chim. Acta, 2015, 884, 61 CrossRef CAS PubMed; (c) R. Meinusch, K. Hormann, R. Hakim, U. Tallarek and B. M. Smarsly, RSC Adv., 2015, 5, 20283 RSC; (d) K. Ohyama, S. Takasago, N. Kishikawa and N. Kuroda, J. Sep. Sci., 2015, 38, 720 CrossRef CAS PubMed; (e) E. Tamashima, T. Hayama, H. Yoshida, O. Imakyure, M. Yamaguchi and H. Nohta, J. Pharm. Biomed. Anal., 2015, 115, 201 CrossRef CAS PubMed; (f) H. Wang and S. V. Olesik, J. Chromatogr. A, 2015, 1379, 56 CrossRef CAS PubMed; (g) Q. Wang, Z. Y. Luo, M. Ye, Y. Z. Wang, L. Xu, Z. G. Shi and L. Xu, J. Chromatogr. A, 2015, 1383, 58 CrossRef CAS PubMed; (h) Q. Wang, M. Ye, L. Xu and Z. G. Shi, Anal. Chim. Acta, 2015, 888, 182 CrossRef CAS PubMed; (i) W. Yin, L. Cheng, H. Chai, R. Guo, R. Liu, C. Chu, J. A. Palasota and X. Cai, Anal. Bioanal. Chem., 2015, 407, 6217 CrossRef CAS PubMed; (j) Y. Zhang, B. Gao, F. An, Z. Xu and T. Zhang, J. Chromatogr. A, 2014, 1359, 26 CrossRef CAS PubMed.
- Y. Yu, M. Z. Rong and M. Q. Zhang, Polymer, 2010, 51, 492 CrossRef CAS.
- G. Fang, Y. Lv, W. Sheng, B. Liu, X. Wang and S. Wang, Anal. Bioanal. Chem., 2011, 401, 3367 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: GC-MS, ESI-HRMS and 1H NMR spectra for organic compounds. See DOI: 10.1039/c6ra04792k |
| ‡ The two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |