Cl-assisted highly efficient synthesis of FePd3 alloys encapsulated in graphite papers: a two stage CVD approach†
Jian Guo‡
a,
Qingyu Ye‡a,
Mu Lana,
Shanling Wangb,
Tian Yua,
Fuhua Gaoa,
Dejiao Hua,
Ping Wanga,
Yi Heb,
Filippo S. Boi*a,
Sijie Zhang*a and
Gang Xiang*a
aCollege of Physical Science and Technology, Sichuan University, Chengdu, China. E-mail: f.boi@scu.edu.cn; sijie.zhang@scu.edu.cn; gxiang@scu.edu.cn
bAnalytical and Testing Centre, Sichuan University, Chengdu, China
First published on 18th April 2016
Abstract
Recently it has been shown that FePd alloys can be encapsulated in graphitic carbon based systems for a better particle dispersion through addition of dichloro-cyclooctadiene palladium to ferrocene. Here we propose an advanced two-stage method which allows the synthesis of very thick deposits of planar rolled-like graphite structures filled with FePd3 alloys as dominant product in the entire reactor. The first stage is used to pyrolyze the Pd-containing hydrocarbon on the top of Si/SiO2 substrates for Pd deposition while the second stage is used for the evaporation and pyrolysis of the Fe-containing precursor (ferrocene) for the FePd alloy formation and encapsulation. Annealing studies also show that no changes in the unit cell of the FePd3 structure are found even after tens of hours at 600–650 °C under Ar/H2 flow. Instead a change in the encapsulated particle shape and spatial arrangement is found. The samples are characterized in detail through scanning and transmission electron microscopy, energy dispersive X-rays, Raman spectroscopy, thermogravimetric analyses, X-ray diffraction and room-temperature magnetometry.
Introduction
In the last decade L10 type magnetic alloys FePt, CoPt, FePd, CoPd have attracted great attention owing to their extremely large magneto-crystalline anisotropy.This intrinsic property is due to the large spin–orbit coupling in the 5d element composing the L10 alloy, and is at the origin of their potentially exceptional magnetic properties (giant coercivity).3–7
Thanks to these properties, these alloys have been considered ideal for applications as exchange coupled nanocomposite magnets and magnetic data recording media1–3 for the achievement of data-densities as high as 1 Tbit in−2.3–7
The crystallographic notation reported in literature identifies the tetragonal arrangement of the L10 type alloys as tP4 with space group P4/mmm3,6,9 (see Person-scheme which combines the typical Bravais lattice and the unit cell informations9).
From the works previously performed in literature on nanoparticles-systems, it seems clear that L10 ferromagnetic alloys exhibit a typical disorder–order transformation, from the defective atomically disordered face-centred cubic (FCC) A1 type structure (low magneto-crystalline anisotropy3,6,9–11) to the ordered L10 type superstructure (high magnetic anisotropy3–9,11,12), in certain conditions. Specifically this type of transformation is found when the temperature is lowered below 1300 °C for FePt, 825 °C for the case of CoPt and 600 °C for the specific cases of FePd and CoPd.7,9
In particular, the presence of magnetic fields during the annealing process has been also reported to play an important role in the phase transformations mentioned above.13,14
In the L10 type structure the cubic symmetry is broken due to the stacking of alternate planes of the 3d elements (Fe) and 5d element (Pt) along the [001] axis.9
Also, it has been reported that the FCC to L10 transition produces two types of crystallographic domains:9 the translational (anti-phase) domains, due to the lowering of the translational symmetry, and the orientational domains (merohedral twins), due to a lowering of the point symmetry.9
The formation of these antiphase domains appears to have a fundamental role in the unique magnetic behaviour of these alloys.
Indeed, numerous reports suggest that the giant coercivity in L10 alloys is due to the presence of the above mentioned anti-phase boundaries which act as pinning sites.9
In the attempt of achieving a complete control of the fabrication mechanism (for the industrial application of such alloys), numerous groups have attempted the fabrication of self-organized magnetic arrays, and films of dispersed L10 particles by numerous methods:
(1) Chemical synthesis,1–3 (2) water based approaches,15 (3) nanosphere lithography, (4) reactive ion etching and thermal deposition,16 (5) E-beam lithography,17,18 (6) microwave irradiation,19 (7) Schlenk line by airless techniques,20 (8) modified polyol process,21 (9) direct chemical synthesis without sintering,22 (10) by combining chemical synthesis and atomic layer deposition23 or (11) by one-pot synthesis.24
However, despite the numerous reports, the accomplishment of a complete long-range order in the whole produced samples requires still sophisticated procedures of preparation.
In particular, several issues related to oxidation after air-exposure, grain growth, coalescence, or sintering are generally found in the annealing stage.7 Another issue of these systems is the substrate dependence that limits the number of applications for which these exceptional ferromagnetic alloys could be used.
In this context, the encapsulation of these alloys within a carbon based material could enhance their stability, ensuring a better protection from the external environment and easier manipulation through annealing, or high pressure methods.
In particular, recent works have shown that films of carbon nanotubes filled with magnetic materials25–33 can be grown directly in situ and used as nano-capsules. The encapsulation process is achieved by evaporation and pyrolysis of single metallocenes in conditions of laminar25–33 or perturbed vapour flow.34,35
These nanostructures can be also grown by evaporation and pyrolysis of mixtures of metallocenes and Cl-containing hydrocarbons. Indeed, the Cl radicals can slow-down the carbon nanotube growth-mechanism through catalyst-etching, and allow the enhancement of the nanotube filling-rate.36–42
Alternatively, other carbon structures can be also considered for potential encapsulation: carbon nano-onions (CNOs),43,44 carbon foam and graphite.45–47
The main challenge that limits the use of a Pd or Pt based catalyst for encapsulation inside carbon-based materials is the carbon solubility.
This is much lower than that generally observed in the widely used Fe-catalyst, where the high carbon solubility allows an easy CNTs or CNOs nucleation (thanks to the formation of Fe3C catalyst phases).
Recently it has been shown that FePd alloys can be encapsulated in carbon based systems, like planar graphite or carbon nanotubes. This encapsulation-process can allow the achievement of better particle dispersion through the addition of a Pd-containing hydrocarbon (dichloro-cyclooctadiene palladium) to ferrocene.47
However, the large difference between the evaporation-temperatures of the two precursors can strongly limit the control of the delivery-rate of the metal and carbon species in the decomposition zone. It was also shown that the encapsulated Fe/CoPd phases could be manipulated through long annealing treatments.47
In this paper we propose an advanced method which allows a better control of the pyrolysis of the metallocene-like precursors (ferrocene and dichloro-cyclooctadiene-palladium) through a two-stage chemical vapour deposition (CVD) approach.
In this approach, the first stage is used to evaporate and pyrolyze the Pd-containing hydrocarbon (at approximately 700 °C) on the top of Si/SiO2 substrates for the achievement of Pd deposition.
After a delay of 3 minutes, the preheater is then set to the temperature of 150 °C for the evaporation of the ferrocene precursor (1 g) on the top of the same substrate.
As a result, our approach shows that very thick deposits of planar rolled-like graphite structures filled with FePd3 alloys could be obtained as dominant product in the entire reactor. The obtained structures are characterized through: scanning electron microscopy (SEM), transmission electron microscopy (TEM), Raman Spectroscopy (RS), X-ray diffraction (XRD), thermogravimetric analyses (TGA) and vibrating sample magnetometry (VSM). Furthermore annealing experiments are performed to study the possible compositional and structural variations of these alloys into the L10 phase.
Experimental
The experiments were performed by using a chemical vapour deposition system composed of a quartz tube of length 1.5 m, inner diameter of approximately 40 mm, one zone electric furnace as shown in the schematic of Fig. 1. | ||
| Fig. 1 Schematic showing the stages of the CVD reaction which yielded large quantities of graphitic carbon papers filled with FePd3 alloys. The decomposition mechanism of ferrocene molecules has been previously studied at different temperatures and the decomposed species shown in (D) have been previously suggested in literature.48 | ||
The experiment was performed as follow: 0.3 g of dichloro-cyclooctadiene-palladium (purchased from Sigma Aldrich 99% purity) were placed on the top of a Si/SiO2 substrate (width of 2 cm and length of 4 cm), and located inside the quartz tube in the deposition zone (zone 2) as shown in Fig. 1A.
1 g of ferrocene (purchased from Sigma Aldrich 98% purity) was then placed in the evaporation zone of the reactor on a quartz boat (zone 1), at a distance of 10 cm from the Si/SiO2 substrate. The entire CVD system was then closed and placed under an Ar flow of 15 mL min−1 (Fig. 1A).
Once the temperature of 990 °C was reached, the furnace was positioned in the deposition zone (through a rail system) as shown in Fig. 1B. This procedure is used to eliminate the extra-carbon content and leave the only Pd catalyst on the substrate–surface (Fig. 1B).
The use of this approach is justified by the fact that dichloro-cyclooctadiene-palladium is found to melt at 210 °C (melting point), and evaporate at 290–300 °C. This temperature is much higher than that necessary for the evaporation of ferrocene (100–200 °C).
We expect that in this first stage the cyclooctadiene molecule will separate from the Pd atoms, and pyrolyse in the regions downstream of the reactor, leaving therefore the Pd catalyst on the Si/SiO2 surface (Fig. 1B).
This mechanism is confirmed by additional SEM and energy dispersive X-ray (EDX) analyses in Fig. S1† of the substrate area shown in Fig. S1 (first inset), and S2A.†
The SEM analyses in Fig. S1 and S2† clearly show the presence of residual Pd catalyst particles on the top of the substrate surface (deposited during the stage 1 of the reaction).
Some examples of the grown structures are also shown in Fig. S2B.† The presence of residual Pd catalyst particles on the substrate surface is also confirmed by the EDX analyses in Fig. S1.† However no Cl residues were found.
Note that the Cl radicals are expected to etch the Pd catalyst surface and form chlorinated carbon clusters with carbon species derived from possible partial pyrolysis of the cyclooctadiene molecule (after detachment from the Pd species); (see ref. 49 for examples about chemical reaction processes in the case of Fe catalyst and Cl radicals and stability of chlorinated carbon clusters).
After a delay of 3 minutes, the preheater was then set to the temperature of 150 °C for the evaporation of the ferrocene precursor and subsequent pyrolysis in the reaction zone as shown in Fig. 1C.
The ferrocene molecule is expected to decompose as follows: Fe(C5H5)2 contains Fe + H2 + CH4 + C5H6 + … Once the reaction time of 1 h (Fig. 1D and E) was reached, the furnace was then removed along the rail system (fast cooling-quench). The sample was extracted once the entire system reached room temperature.
The characterization was performed with the following techniques: (1) TEM with a 200 kV American FEI Tecnai G2 F20. A small quantity of the sample was firstly dispersed in ethanol, and then transferred through the use of a pipette on the top of the TEM-grid. The TEM grid consisted of copper with a holey carbon film on the top.
(2) XRD with a Empyrean Panalytical (S/N: DY1588, Cu Kα source with λ = 0.154 nm), with 0.013° per step (step-size) and 30 s as time per step.
(3) Vibrating sample magnetometry (VSM) at room temperature with a VSM 2.5 tesla electromagnet East Changing 9060, at the magnetic field of 1.3 tesla, sensitivity 2 mV and time constant 200 ms. These measurements are performed through a static approach with the following steps: step 1: the field is applied to the sample, step 2: the field is stabilized, step 3: the signal is measured. These steps are repeated for different field values for the hysteresis measurement.
(4) TGA with a Mettler Toledo at the temperature of 800 °C. The heating rate was 10° min−1 from 30 °C to 800 °C. The temperature was then kept constant for 1.5 h.
(5) SEM and EDX with a JSM-7500F at 5–20 kV.
(6) Raman spectroscopy with an Horiba Jobin Yvon HR Evolution with power 15 mW with approximately 40% output.
Results and discussion
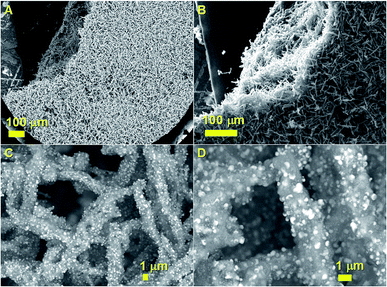
The morphology of the graphitic carbon papers was firstly revealed by SEM. Fig. 2 shows typical examples of scanning electron micrographs taken with an increasing level of detail of the as grown graphitic carbon papers filled with FePd3 crystals. In particular it can be immediately noticed from the micrographs in Fig. 2A and B that the thickness of the deposit corresponds to that of 0.2 mm, which underlines the suitability of this method for industrial mass production purposes. The higher detail investigations of the produced nanostructures revealed that the graphitic carbon papers have a rolled-like morphology as shown in Fig. 2C and D with backscattered electrons investigations. The FePd3 particles can be clearly observed thanks to the atomic number differences with the carbon in the rolled papers. EDX analyses were also used to investigate the element ratio. Note that, due to the contribution of the elements in the neighbour alloy particles within the graphitic structures, the measurements on single particles could not be performed. However it seems clear from the atomic% values and weight% values in Fig. S3† that a possible ratio of Fe (1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd (3) is present. Also we will show later that this composition is confirmed by XRD and Rietveld analyses.
Pd (3) is present. Also we will show later that this composition is confirmed by XRD and Rietveld analyses.
The cross-section of the as grown papers was investigated through TEM. The TEM micrographs revealed a high quantity of FePd3 particles encased in the carbon layers. Typical examples of the cross-sectional morphologies of the papers are shown in Fig. 3A–C. The particles encapsulated in the graphite layers are shown with higher detail in Fig. 3D. In the as grown sample is interesting to notice that many particles form large agglomerations (Fig. 3D). The graphitic structure of the carbon papers was firstly confirmed by Raman Spectroscopy investigations in Fig. 4 which revealed the presence of three bands in the region of 1150 cm−1, 1259 cm−1 and 1568 cm−1 respectively. The bands at approximately 1150–1259 cm−1 (D band) can be associated to the disorder-induced scattering arising from imperfections or loss of hexagonal symmetry in the carbon structure. The band observed at 1568 cm−1 (G band) can be assigned to the Raman 2E2g mode which is generally observed in graphite like materials.
 | ||
| Fig. 3 TEM micrographs showing the morphology of the as grown rolled-like carbon papers filled with FePd3 alloys. | ||
Further investigations of the carbon papers structure were then performed with XRD and analysed through Rietveld refinements methods. The typical XRD diffractogram of the as produced sample is shown in Fig. 5 and reveals the presence of three main peaks which could be attributed to the 111, 200 and 220 reflections of FePd3 with space group Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The measured unit cell parameters were a = b = c = 0.38 nm. The graphitic nature of the carbon papers was also confirmed by the presence of small 002 reflections of graphite with space group P63/mmc. In the attempt to investigate possible phase transformations of the FePd3 phase into the ordered tetragonal FePd alloy, annealing treatments at the temperature of 600–650 °C were considered. Interestingly as shown in Fig. 6, in this case no changes are observed in the structure of the FePd3 alloy after annealings performed for 3 h (Fig. 6A) and 10 h (Fig. 6B) respectively. Rietveld refinement investigations were then considered to compare the unit cell of the annealed FePd3 phase with that in the as grown sample.
m. The measured unit cell parameters were a = b = c = 0.38 nm. The graphitic nature of the carbon papers was also confirmed by the presence of small 002 reflections of graphite with space group P63/mmc. In the attempt to investigate possible phase transformations of the FePd3 phase into the ordered tetragonal FePd alloy, annealing treatments at the temperature of 600–650 °C were considered. Interestingly as shown in Fig. 6, in this case no changes are observed in the structure of the FePd3 alloy after annealings performed for 3 h (Fig. 6A) and 10 h (Fig. 6B) respectively. Rietveld refinement investigations were then considered to compare the unit cell of the annealed FePd3 phase with that in the as grown sample.
 | ||
| Fig. 5 XRD diffractogram (black line) and Rietveld refinement (red line) of the as grown carbon papers filled with FePd3 alloys. | ||
Interestingly no remarkable changes were observed in the unit cell parameters which were found to be a = b = c = 0.38 nm. Furthermore a decrease in the intensity of the carbon peak is observed with the increase of the annealing time suggesting therefore a possible decrease in the number of graphitic layers.
Further TEM investigations of the graphitic carbon papers annealed for 10 h revealed a change in morphology of the encapsulated particles. As shown in Fig. 6C in this case the shape of the particles appears to have a more spherical definition which is different from the faceted-like morphology observed in Fig. 3. A typical example of a single rolled graphite paper obtained after annealing is shown in Fig. 6C. Interestingly after annealing the particles appear also to possess a spherical-like arrangement with a more defined distribution.
Thermogravimetric analyses were then considered for evaluating the metal content in the carbon papers. As shown in Fig. 7, the observed metal content corresponded to 66.4% while the rest of the weight loss was associated to the carbon paper. The weight loss of 33.6% can be associated to the decomposition process of graphitic carbon. An unusual change of slope is also observed in the first part of the TGA curve at approximately 650 °C, as shown in Fig. 7A. This change of slope could be associated to the presence of strained regions in the graphitic films which would then decompose in different timescales. In Fig. 7B a high detail of the final part of the TGA measurement is shown. Another change of slope is also observed at approximately 800 °C. This slope-change can be explained considering that the TGA measurement was performed in two stages: a first stage involving heating until the temperature of approximately 800 °C and a second stage in which the temperature was kept constant for 1.5 h to ensure the complete decomposition of the graphitic carbon. The results extracted with the TGA measurements are also in agreement with the Raman spectroscopy measurement shown above. The investigation of the magnetic properties of the as grown carbon papers with VSM was then considered. As shown in Fig. 8 a saturation magnetization of 16.6 emu g−1 (16.6 A m2 kg−1) and a coercivity of 56 Oe (4456 A m−1) were found (see inset in Fig. 8). These values can be explained considering that the FePd3 alloy is a soft magnetic phase with low magneto-crystalline anisotropy.47
 | ||
| Fig. 7 TGA analyses of the as grown carbon papers filled with FePd3 alloys showing a metal% of 66.44 after complete decomposition of the graphitic carbon. | ||
 | ||
| Fig. 8 VSM analyses of the as grown carbon papers filled with FePd3 alloys. The coercivity is shown with higher detail in the inset. | ||
The measured coercivity is much lower than that of 700–900 Oe (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706–71
706–71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622 A m−1) measured by Boi et al. for planar graphitic structures and carbon nanotubes filled with FePd3.47 Furthermore the measured coercivity is also much lower with respect to those reported in previous literature reports on FePd films by Hung-Pin Lin et al.:10 309 Oe (24
622 A m−1) measured by Boi et al. for planar graphitic structures and carbon nanotubes filled with FePd3.47 Furthermore the measured coercivity is also much lower with respect to those reported in previous literature reports on FePd films by Hung-Pin Lin et al.:10 309 Oe (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590 A m−1), 159 Oe (12
590 A m−1), 159 Oe (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 653 A m−1) and 118 Oe (9390 A m−1); and on un-annealed samples by K. Watanabe et al.: 630 Oe (50
653 A m−1) and 118 Oe (9390 A m−1); and on un-annealed samples by K. Watanabe et al.: 630 Oe (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135 A m−1).21 The measured coercivity was however higher with respect to those of 10–30 Oe (796–2387 A m−1) observed by N. T. T. Van et al.,6 H. Loc Nguyen et al.19 and K. Mori et al.20 In the last two cases a superparamagnetic behaviour of the fcc FePd phases was reported. Instead the measured saturation magnetization is comparable to that of 8 emu g−1 (8 A m2 kg−1) reported by H. Loc Nguyen et al. and that of 19 emu g−1 (19 A m2 kg−1) reported by K. Mori et al.20
135 A m−1).21 The measured coercivity was however higher with respect to those of 10–30 Oe (796–2387 A m−1) observed by N. T. T. Van et al.,6 H. Loc Nguyen et al.19 and K. Mori et al.20 In the last two cases a superparamagnetic behaviour of the fcc FePd phases was reported. Instead the measured saturation magnetization is comparable to that of 8 emu g−1 (8 A m2 kg−1) reported by H. Loc Nguyen et al. and that of 19 emu g−1 (19 A m2 kg−1) reported by K. Mori et al.20
Conclusion
In conclusion we demonstrated an advanced method which allows the synthesis of very thick deposits of planar rolled-like graphite structures filled with FePd3 alloys as dominant product in the entire reactor. In this approach the first stage is used to pyrolyze the dichloro-cyclooctadiene-palladium hydrocarbon on the top of Si/SiO2 substrates for the deposition of Pd particles on the Si-substrate and removal of carbon feedstock present in the hydrocarbon. After a delay of 3 minutes in the second stage the preheater is then used for the evaporation of the ferrocene precursor on the top of the same substrate for the deposition of the Fe species and subsequent formation of FePd3 alloys encapsulated in graphitic like structures. Furthermore no changes in the composition of the produced alloy were observed after multiple annealing experiments at 600–650 °C.Acknowledgements
We acknowledge Prof. Gong Min for his continuous support in this research work. We are also grateful for the financial support from the National Natural Science Foundation of China (Grant No. 11404227).References
- H. Zeng, J. Li, J. P. Liu, Z. L. Wang and S. Sun, Nature, 2002, 420, 395–398 CrossRef CAS PubMed.
- S. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989–1991 CrossRef CAS PubMed.
- D. Alloyeau, C. Ricolleau, C. Mottet, T. Oikawa, C. Langlois, Y. Le Bouar, N. Braidy and A. Loiseau, Nat. Mater., 2009, 8, 940–946 CrossRef CAS PubMed.
- K. D. Belashchenko and V. P. Antropov, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 144402 CrossRef.
- K. Sato, B. Bian and Y. Hirotsu, J. Appl. Phys., 2002, 91, 8516 CrossRef CAS.
- N. T. T. Van, T. T. Trung, N. H. Nam, N. D. Phu, N. H. Ha and N. H. Luong, Eur. Phys. J.: Appl. Phys., 2013, 64, 10403–10406 CrossRef.
- O. Gutfleisch, J. Lyubina, K. H. Muller and L. Shultz, Adv. Eng. Mater., 2005, 7, 208–212 CrossRef CAS.
- M. Ohtake, S. Ouchi, F. Kirino and M. Futamoto, J. Appl. Phys., 2012, 111, 07A708 Search PubMed.
- D. E. Laughlin, K. Srinivasan, M. Tanase and L. Wang, Scr. Mater., 2005, 53, 383–388 CrossRef CAS.
- H. P. Lin and J. C. Kuo, Mater. Lett., 2011, 65, 3537–3539 CrossRef CAS.
- V. G. Myagkov, V. S. Zhigalov, B. A. Belyaev, L. E. Bykova, L. A. Solovyov and G. N. Bondarenko, J. Magn. Magn. Mater., 2012, 324, 1571–1574 CrossRef CAS.
- L. Wang, Z. Fan, A. G. Roy and D. E. Laughlin, J. Appl. Phys., 2004, 95, 7483–7485 CrossRef CAS.
- D. S. Li, H. Garmestani, Y. Shi-shen, M. Elkawni, M. B. Bacaltchuk, H. J. Schneider-Muntau, J. P. Liu, S. Saha and J. A. Barnard, J. Magn. Magn. Mater., 2004, 281, 272–275 CrossRef CAS.
- K. Tanaka, T. Ichitsubo and M. Koiwa, Mater. Sci. Eng., A, 2001, 312, 118 CrossRef.
- N. Sakulchaicharoen, D. M. O'Carroll and J. E. Herrera, J. Contam. Hydrol., 2010, 118, 117–127 CrossRef CAS PubMed.
- C. C. Yu, Y. D. Yao and S. C. Chou, J. Magn. Magn. Mater., 2007, 310, 2333–2335 CrossRef CAS.
- Y. J. Chen, T. L. Huang, J. Z. Shi, J. Deng, J. Ding, W. M. Li, S. H. Leong, B. Y. Zong, H. Yu Yu Ko, S. B. Hua and J. M. Zhao, J. Magn. Magn. Mater., 2012, 324, 264–268 CrossRef CAS.
- W. M. Li, Y. Yang, Y. J. Chen, T. L. Huang, J. Z. Shi and J. Ding, J. Magn. Magn. Mater., 2012, 324, 1575–1580 CrossRef CAS.
- H. Loc Nguyen, L. E. M. Howard, S. R. Giblin, B. K. Tanner, I. Terry, A. K. Hughes, I. M. Ross, A. Serres, H. Burckstummer and J. S. O. Evans, J. Mater. Chem., 2005, 15, 5136–5143 RSC.
- K. Mori, Y. Kondo and H. Yamashita, Phys. Chem. Chem. Phys., 2009, 11, 8949–8954 RSC.
- K. Watanabe, H. Kura and T. Sato, Sci. Technol. Adv. Mater., 2006, 7, 145–149 CrossRef CAS.
- Y. Yu, P. Mukherjee, Y. Tian, X.-Z. Li, J. E. Shield and D. J. Sellmyer, Nanoscale, 2014, 6, 12050–12055 RSC.
- A. C. Johnston-Peck, G. Scarel, J. Wang, G. N. Parsons and J. B. Tracy, Nanoscale, 2011, 3, 4142–4149 RSC.
- Y. Yu, K. Sun, Y. Tian, X.-Z. Li, M. J. Kramer, D. J. Sellmyer, J. E. Shield and S. Sun, Nano Lett., 2013, 13, 4975–4979 CrossRef CAS PubMed.
- H. Terrones, F. López-Urías, E. Muñoz-Sandoval, J. A. Rodríguez-Manzo, A. Zamudio and A. L. Elías, et al., Solid State Sci., 2006, 8, 303 CrossRef CAS.
- F. S. Boi, S. Maugeri, J. Guo, M. Lan, S. Wang, J. Wen, G. Mountjoy, M. Baxendale, G. Nevill, R. M. Wilson, Y. He, S. Zhang and G. Xiang, Appl. Phys. Lett., 2014, 105, 243108 CrossRef.
- A. Leonhardt, M. Ritschel, M. Elefant, D. N. Mattern, K. Biedermann, S. Hampel, C. Muller, T. Gemming and B. Buchner, J. Appl. Phys., 2005, 98, 074315 CrossRef.
- S. Hampel, A. Leonhardt, D. Selbmann, K. Biedermann, D. Elefant, C. Muller, T. Gemming and B. Buchner, Carbon, 2006, 44, 2316 CrossRef CAS.
- A. Leonhardt, M. Ritschel, R. Kozhuharova, A. Graff, T. Muhl, R. Huhle, I. Monch, D. Elefant and C. M. Schneider, Diamond Relat. Mater., 2003, 12, 790 CrossRef CAS.
- A. Morelos-Gomez, F. Lopez-Urias, E. Munoz-Sandoval, C. L. Dennis, R. D. Shull, H. Terrones and M. Terrones, J. Mater. Chem., 2010, 20, 5906 RSC.
- C. Prados, P. Crespo, J. M. Gonzalez, A. Hernando, J. F. Marco, R. Gancedo, N. Grobert, M. Terrones, R. M. Walton and H. W. Kroto, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 113405 CrossRef.
- J. F. Marco, J. R. Gancedo, A. Hernando, P. Crespo, C. Prados, J. M. Gonzalez, N. Grobert, M. Terrones, D. R. M. Walton and H. W. Kroto, Hyperfine Interact., 2002, 139, 535 CrossRef.
- S. Karmakar, S. M. Sharma, M. D. Mukadam, S. M. Yusuf and A. K. Sood, J. Appl. Phys., 2005, 97, 054306 CrossRef.
- F. S. Boi, G. Mountjoy, R. M. Wilson, Z. Luklinska, L. J. Sawiak and M. Baxendale, Carbon, 2013, 64, 351–358 CrossRef CAS.
- F. S. Boi, G. Mountjoy and M. Baxendale, Carbon, 2013, 64, 516 CrossRef.
- W. Wang, K. Wang, R. Lv, W. J. Zhang, X. Kang and F. Chang, et al., Carbon, 2007, 45, 1105–1136 CrossRef.
- R. Lv, S. Tsuge, X. Gui, K. Takai, F. Kang, T. Enoki, J. Wei, J. Gu, K. Wang and D. Wu, Carbon, 2009, 47, 1141 CrossRef CAS.
- X. Gui, K. Wang, W. Wang, J. Wei, X. Zhang, R. Lv, Y. Jia, Q. Shu, F. Kang and D. Wu, Mater. Chem. Phys., 2009, 113, 634–637 CrossRef CAS.
- R. Lv, F. Kang, W. Wang, J. G. J. Wei, K. Wang and D. Wu, Carbon, 2007, 45, 1433 CrossRef CAS.
- R. Lv, A. Cao, F. Kang, W. Wang, J. Wei and J. Gu, J. Phys. Chem. C, 2007, 111, 11475 CAS.
- R. Lv, F. Kang, J. Gu, X. Gui, J. Wei, K. Wang and D. Wu, Appl. Phys. Lett., 2008, 93, 223105 CrossRef.
- J. Guo, M. Lan, S. Wang, Y. He, S. Zhang, G. Xiang and F. S. Boi, Phys. Chem. Chem. Phys., 2015, 17, 18159 RSC.
- P. Z. Si, Z. D. Zhang, D. Y. Geng, C. Y. You, X. G. Zhao and W. S. Zhang, Carbon, 2003, 41, 247–251 CrossRef CAS.
- A. S. Rettenbacher, B. Elliott, J. S. Hudson, A. Amirkhanian and L. Echegoyen, Chem.–Eur. J., 2006, 12, 376–387 CrossRef PubMed.
- M. Inagaki, J. Qiu and Q. Guo, Carbon, 2015, 87, 128–152 CrossRef CAS.
- S. T. Mitchell, N. Frese, A. Golzhauser, A. Bowers and K. Sattler, Carbon, 2015, 95, 434–441 CrossRef CAS.
- F. S. Boi, J. Guo, M. Lan, G. Xiang, S. Wang, J. Wen and S. Zhang, Carbon, 2015, 95, 634–639 CrossRef CAS.
- U. Weissker, S. Hampel, A. Leonhardt and B. Buchner, Materials, 2010, 3, 4387–4427 CrossRef CAS.
- S. Dimovski, A. Nikitin, H. Ye and Y. Gogotsi, J. Mater. Chem., 2004, 14, 238–243 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04777g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |