Porphyrin dimers as donors for solution-processed bulk heterojunction organic solar cells†
Abstract
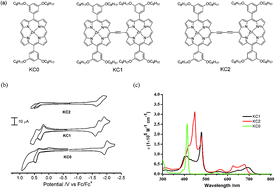
We synthesize a novel class of porphyrin dimers consisting of two zinc-metalated porphyrin units covalently linked through ethynyl (KC1) or butadiyne (KC2) group as electron donors blended with [6,6]-phenyl C70 butyric acid methyl ester (PC70BM) as electron acceptor for the fabrication of organic bulk heterojunction (BHJ) solar cells. The solution processed BHJ solar cells with porphyrin dimers:PC70BM show superior performance than those based on their parent monomer due to much broader light absorption spectra. It is found that devices with KC2 : PC70BM blend in the weight ratio of 1 : 4 exhibit higher power conversion efficiency (PCE) over those with KC1:PC70BM blend. When the KC2:PC70BM blend is processed from solvent containing 3% v/v 1-chloronaphathalene (CN), the PCE of the solar cell is improved to 2.03% from 1.73%. The superior performance and the performance improvement of the solar cells with KC2:PC70BM are due to the high short circuit current density (Jsc) resulting from a higher effective device mobility and a lower series resistance. The study provides a useful guideline for designing porphyrin based donor materials for optoelectronic device application.

 Please wait while we load your content...
Please wait while we load your content...