Heterogeneous Fenton-like degradation of methyl blue using MCM-41-Fe/Al supported Mn oxides
Abstract
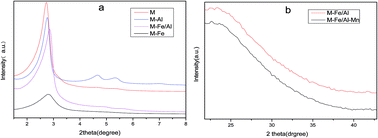
Conventional heterogeneous catalysts often suffer from the constraints of high Fe3+ leaching concentrations and an amorphous appearance, which can affect the stability of the catalysts. We prepared uniform and spherical M-Fe/Al at room temperature. Furthermore, this material was supported with Mn oxides by an impregnation method to accelerate the degradation process of methyl blue. XRD, TEM, BET, and zeta potential measurements were employed to characterize the catalyst. In our work, M-Fe/Al–Mn showed a better methyl blue degradation efficiency than M-Fe/Al in the presence of H2O2. 93.3% discoloration was achieved by using M-Fe/Al–Mn within 200 min at 313 K. This catalyst performed better than M-Fe/Al. This could mainly be attributed to the increased adsorption effect of the Mn oxides. Furthermore, M-Fe/Al–Mn also showed good recyclability and stability over 5 cycles. Moreover, the Fe3+ leaching concentration was negligible (below 0.5 mg L−1) in our work.

 Please wait while we load your content...
Please wait while we load your content...