Electrochemiluminescent molecular logic gates based on MCNTs for the multiplexed analysis of mercury(II) and silver(I) ions†
Haiyun Liua,
Lina Zhangb,
Meng Lia,
Mei Yana,
Mei Xuec,
Yan Zhanga,
Min Sua,
Jinghua Yu a and
Shenguang Ge*a
a and
Shenguang Ge*a
aKey Laboratory of Chemical Sensing & Analysis in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, P. R. China. E-mail: chm_gesg@ujn.edu.cn; Tel: +86-531-82767161
bShandong Provincial Key Laboratory of Preparation and Measurement of Building Materials, University of Jinan, Jinan 250022, P. R. China
cCollege of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Jinan, 250014, P. R. China
First published on 2nd March 2016
Abstract
In this paper, logic gates with electrochemiluminescence (ECL) signal as outputs were constructed based on the use of the thymine (T)-rich (S1) or cytosine (C)-rich (S2) oligonucleotides for the selective analysis of mercury ions (Hg2+) or silver ions (Ag+), respectively. Firstly, Ru-silica (Ru(bpy)32+-doped silica) labeled S1 and S2 were absorbed onto multiwalled carbon nanotubes (MCNTs) to form MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex, which result in the quenching of the ECL of Ru-silica via the energy-transfer and electron-transfer process. Upon the MCNTs/S1/S2 interaction and coordination chemistry of Hg2+ bridge thymine bases and Ag+ specifically bridge cytosine bases, OR, INHIBIT, and NOR logic gates were designed to operate the ECL of Ru-silica nanoparticles. The ECL signal changed according to different input combinations, and the combinatorial logic gates (OR and INHIBIT) provided a beneficial approach for multiplex analysis. The proposed logic gates may have a great potential in further DNA circuits and advanced sensors for the identification of multiple targets in complex chemical environments.
1 Introduction
Molecular logic gates are good candidates for the applications of various chemical systems that mimic logic operations, including sensing, biotechnology, nanomedicine and diagnostics.1,2 Consequently, extensive and ongoing research in mimicking Boolean logic gates has been motivated. Until now, molecular logic gates have been constructed from various materials, for instance nucleic acids, enzymes, and other small molecules.3–6 DNA, a special biological molecular, possessing straight forward sequence-specific hybridization7 and the ability to specifically capture certain target molecules (e.g., proteins, small molecules, and metal ions),8,9 exhibits distinct advantages in designing addressable DNA logic gates.10–12 For example, Willner and co-workers constructed a series of fluorescent DNA logic gates which were driven by Mg2+, oligonucleotides, protein or small molecules.12–15 Wang and co-workers fabricated a colorimetric logic gate utilizing Pb2+ or K+ as inputs.16 However, these reported logic gates often suffer from relatively complicated handling procedures, not portable, and lower sensitivity. Recently, Crooks report an individual microelectrochemical logic gates first based on the principles of bipolar electrochemistry.17 Li and co-workers reported the fabrication of logic gates with electrochemiluminescence (ECL) signals as their outputs18 owing to its inherent features, such as low cost, rapid determination, wide range of analytes and high sensitivity.19 Therefore, ECL signal was also employed as outputs in this work.Mercury ions (Hg2+) and silver ions (Ag+), act as severe environmental pollutants, are two common toxic heavy metal ions pollutants causing serious health problems with very limited quantity present.20,21 For Hg2+, it does harm to brain, heart, stomach, intestine, and kidney,22,23 while Ag+ can inactivate sulfhydryl enzymes and accumulate in the body.21 Thus, new method with high selectivity and sensitivity to such ions in water or food resources was of vital importance. Various ions could form stable complexes by bridging specific nucleotide bases. For example, two specific mismatched base pairs of thymine–Hg2+–thymine (T–Hg2+–T) and cytosine–Ag+–cytosine (C–Ag+–C) have been reported and shown potential in the design of sensing platforms for Hg2+ and Ag+.24 Based on the construction of T–Hg2+–T and C–Ag+–C base pair, a large number of sensors have been developed for the detection of Hg2+ and Ag+.25,26 Responsely, the construction of DNA molecular logic gates was proposed, which produced ECL signals as their outputs, having the advantages of versatility, low background and simplified optical setup.18,27
Recently, on-going research of the quenching of Ru(bpy)32+/tripropylamine (TPA) ECL system has attracted particular attention, which was critically important to extend its application with improved performance.28,29 For example, the quenching mechanism of Ru(bpy)32+/TPA ECL system by ferrocene and phenol, has been developed and applied in sequence-specific DNA detection.30–32 However, effective solutions for the above studies are limited, and new methods or materials that could quench the ECL of Ru(bpy)32+/TPA were urgent need to develop new ECL-based applications. Currently, Richter et al. have reported that compounds such as phenols and benzoquinones could quench the ECL of the Ru(bpy)32+/TPA system via energy transfer.33 It has been reported that carbon based nanomaterials have been adapted to achieve efficient resonance energy transfer.34 For example, the carbon nanotubes (CNT) could quench fluorescence due to energy transfer or electron transfer between fluorophores and the CNT.35,36 Meanwhile, oxygen-containing groups could be easily produced at the end or defects in the sidewall of the CNT, especially those previously subjected to purification and cutting.37,38 Relying on the above preliminary work, the study emerged on that oxygen-containing groups at the surface of the multiwall carbon nanotubes (MCNTs) quenched the ECL of Ru(bpy)32+/TPA system via energy-transfer and electron-transfer process.39
In this study, a new platform was employed to construct logic gates by taking advantage of the unique MCNTs/aptamer complex and the high binding specificity of aptamers. Ru-silica (Ru(bpy)32+-doped silica) labeled T-rich (S1) and C-rich (S2) oligonucleotides were firstly absorbed onto the MCNTs to form the MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex, leading to the quenching of the ECL signal of Ru-silica. MCNTs show large surface area and high electronic conductivity. MCNTs could be applied to improve the performance of the modified electrode, since MCNTs increased the mechanical strength and conductivity of the logic gates. And that, MCNTs quenched the ECL of Ru(bpy)32+/TPA system, effectively reduced the background and improved the detection sensitivity. Different logic gates, including OR, INHIBIT, and NOR, were designed to regulate the ECL signal based on MCNTs/S1/S2 interaction and coordination chemistry of T–Hg2+–T and C–Ag+–C. Meanwhile, the integration of OR and INHIBIT logic gates provided an attractive method for logic sensing applications where multiple target molecules were present. The developed logic gates might provide a new approach for multiplex analysis and nanobiomedical devices for multiple input chemicals.
2 Materials and methods
2.1 Reagents
All oligonucleotides were synthesized and purified from Shanghai Linc-Bio Science Co. Ltd. (Shanghai, China). 6-Mercapto-1-hexanol (MCH) and glycine were purchased from Nanoport. Co. Ltd. (Shenzhen, China). Tris(2,2′-bipyridyl)dichlororuthenium(II)hexahydrate (Ru(bpy)32+), TPA, and 3-aminopropyltrimethoxysilane (APS) were obtained from Alfa Aesar China Ltd. Silver nitrate (AgNO3), mercury nitrate (Hg(NO3)2) were obtained from Sigma (St. Louis, MO, USA). Tetraethyl orthosilicate (TEOS), glutaraldehyde solution (GA, 25%), Triton X-100 (TX-100), n-hexanol, and cyclohexane were purchased from Nektar (Huntsville, AL). MCNTs (CVD method, purity > 95%) were purchased from Nanoport. Nitric acid (HNO3), and sulfuric acid (H2SO4) were purchased from Shanghai Chemical Reagent Company (Shanghai, China). All chemicals and solvents were analytical grade available and used as received. The sequences of oligonucleotides are presented with the following sequences:S1: 5′-SH-(CH2)6-TTC TTT CTT CCC TTG TTT GTT-(CH2)6-NH2-3′
S2: 5′-SH-(CH2)6-CTC TCT TCT CTT CAA AAA ACA ACA CAA CAC AC-(CH2)6-NH2-3′
The ultra-pure water was obtained from a Lichun water purification system (≥18 MΩ cm, Jinan, China) and used throughout. The buffers involved in this work are as follows: hybridization buffer was 10 mM phosphate buffered saline (PBS, pH 7.4) with 0.25 mM NaCl. Buffer for ECL was 10 mM Tris–HCl buffer (pH 7.4) containing 0.1 M KCl, and 1.0 × 10−5 M TPA was used as the coreactant.
2.2 Apparatus
The ECL measurements were conducted using a flow injection luminescence analyzer (IFFM-E, Xi'an Remex Electronic Instrument High-Tech Ltd., Xi'an, China) and the voltage of the photomultiplier tube (PMT) was set at 800 V. Cyclic voltammetric measurements (CVs) were performed with a CHI 760D electrochemical workstation (Shanghai CH Instruments, China). Transmission electron microscopy (TEM) image of Ru-silica nanoparticles was obtained with a Hitachi H-800 microscope (Japan). Scanning electron microscope (SEM) image of MCNTs was obtained by a QUANTA FEG 250 thermal field emission SEM (FEI Co., USA). All experiments were carried out with a conventional three-electrode system with the modified GCE (3 mm in diameter) as the working electrode (WE), a platinum counter electrode (CE) and an Ag/AgCl (sat. KCl) reference electrode (RE).2.3 Synthesis of ECL probe
The amino-group functionalized Ru-silica nanoparticles were synthesized according to the previous method.40 Briefly, the water-in-oil microemulsion was prepared firstly by mixing 0.177 mL of TX-100, 0.75 mL of cyclohexane, 0.18 mL of n-hexanol, and 34 μL of water. In the presence of 10 μL of TEOS, a polymerization reaction41 was started reacting by adding 0.0200 g of (Ru(bpy)32+), 1.80 mL of water and 60 μL of NH4OH, and the reaction was kept for 24 h. When the reaction was completed, the Ru-silica nanoparticles were isolated by acetone. To remove any surfactant molecules, the product was centrifuged and then washed with ethanol and water several times. After that, the Ru-silica nanoparticles were re-dispersed in the microemulsion, and 10 μL of TEOS, 6 μL of NH4OH were successively added under stirring, and the mixture was kept stirring for 12 h. Afterwards, 10 μL of APS and 6 μL of NH4OH were added to the solution, and then kept stirring for another 12 h. After the reaction was completed, 2.5 mL acetone was added to break the microemulsion and recover the particles. To remove surfactant molecules from the surface of the particles, the obtained solution was centrifuged and washed by ethanol and water for several times, separately. The Ru-silica nanoparticles were dried under vacuum conditions. The thickness of the post silica coating was controlled by the amount of added TEOS and the aging time of the polymerization reaction. After that, 100 μL of 2 μM aptamer was incubated with 200 μL of Ru-silica nanoparticles, followed by adding 20 μL of 2.5% GA solution. The aptamer was activated with an acetate buffer (pH 5.2) and 1.5 μL of 10 mM TCEP for 1 h. The mixture was stirred at room temperature for 4 h. Thus, the Ru-silica nanoparticles labeled aptamer (Ru-silica-aptamer) was obtaind.2.4 Logic assay
MCNTs were firstly oxidized by the reported method,39 and then were mixed with Ru-silica labeled S1 and Ru-silica labeled S2 for 30 min to form MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex. Subsequently, the complex was mixed with different possible input combinations for 60 min. Finally, the ECL measurements were performed after addition of 1.0 × 10−5 M TPA at room temperature and the potential swept from 0.5 to 1.2 V with scanning rate of 100 mV s−1.3 Results and discussion
3.1 Characterization of MCNTs and Ru-silica nanoparticles
Fig. 1A showed the SEM image of MCNTs with the diameter of individual MCNT about 30–40 nm, which were employed to quench the ECL of Ru(bpy)32+/TPA according to the reported literature.39 Fig. 1B was the TEM image of Ru-silica nanospheres, with a diameter about 30 nm. Fig. 1C showed the CVs and corresponding ECL signal of the Ru-silica/TPA system with the bare and the treated-MCNTs modified electrodes, respectively. As shown in Fig. 1C, it was clearly observed that the current obtained with the MCNTs modified electrode was even slightly larger than the bare electrode. However, the ECL intensity was suppressed when using MCNTs modified electrode in comparison with the bare electrode. The results indicated that the treated-MCNTs made great contribution to the quenching of ECL at the electrode.39 To further investigate the successful preparation of the Ru-silica nanospheres, an EDX was employed for confirmation. Fig. 1D showed the EDX of the Ru-silica nanoparticles, indicating that the composites were composed of O, Si, and Ru elements.3.2 Principle of the logic operation
In this study, the construction of molecular logic gates producing ECL signal as outputs, which were based on the T-rich or C-rich oligonucleotides for the selective analysis of Hg2+ and Ag+ using energy or electron transfer-quenching path, was reported. Our logic protocols were based on the following principles. Firstly, T–Hg2+–T and C–Ag+–C coordination chemistry resulted in Hg2+- or Ag+-stabilized hybridization of oligonucleotides with T–T or C–C mismatches, respectively.18,42,43 Secondly, intense ECL quenching was obtained when the Ru-silica nanoparticles were in close proximity to the surface of the MCNTs, the decrease in their luminescence is attributed to electron-transfer quenching of the MCNTs by the ions bound to the thymine or cytosine bases. While strong ECL signals were generated when Ru-silica nanoparticles were removed from the surface of MCNTs. Thirdly, efficient and stable quenching of ECL of Ru-silica nanoparticles by the treated MCNTs enabled the utilization of different input combinations to activate logic gates, as well as the implementation of Ru-silica ECL as readout signals for logic gate operations.3.3 OR gate
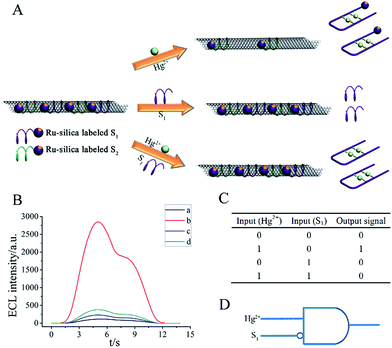
OR gate is one of the basic gates from which all other functions can be constructed. The working principle of MCNTs/aptamer system to perform an OR logic gate was schematically represented in Fig. 2A. As the basis of OR logic gates, Ru-silica labeled S1 and S2 were firstly mixed with MCNTs solution to form MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex, in which the ECL signal of Ru-silica was quenched via energy-transfer or electron-transfer process by MCNTs. Fig. 2B showed the ECL intensity of the MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex at different conditions. In the absence of Hg2+ and Ag+, nearly no ECL signal could be obtained owing to the high quenching efficiency of MCNTs. However, a significant ECL enhancement was observed after the addition of Hg2+ or Ag+ to MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex. The reason might be that the formation of rigid hairpin structure resulted in the detachment of the Ru-silica labeled S1 or Ru-silica labeled S2. When both Hg2+ and Ag+ were present, higher ECL intensity was obtained since coexisting Hg2+ and Ag+ formed more stem-loop structures and more Ru-silica was detached from the surface of MCNTs. The restoring ECL of the MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex by Hg2+ and Ag+ could be employed to design an OR logic gate. Herein, an ECL intensity of 1050 a.u. was defined as the threshold value, which relied on the ECL signal corresponding to the detection limits of Hg2+ and Ag+. The ECL intensities higher than 1050 a.u. were defined as output 1 and the ECL intensities lower than 1050 a.u. were defined as output 0. Correspondingly, the truth table of the resulting OR logic gate was given in Fig. 2C and D was the symbol of the OR gate.The OR gate possesses the unique property that it produces high output when any of the inputs is in the high state, and the output is low when all the inputs are all in the low state.18 Accordingly, it was employed to perform control experiments to realize the selectivity of the logic system toward Hg2+ and Ag+, respectively. It was observed that the system showed appreciable ECL signal in response to Hg2+ and Ag+, but hardly exhibited substantial responses to other metal ions (Fig. S1 in ESI†).
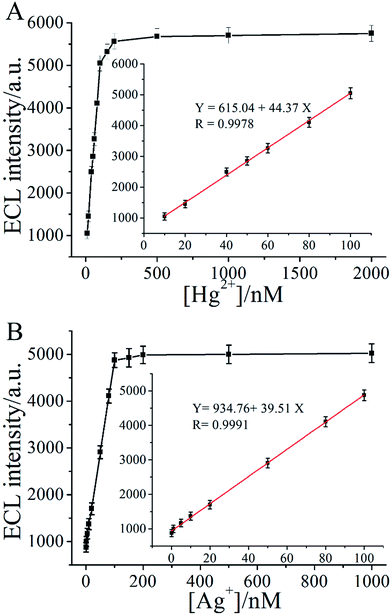
Fig. 3A and B illustrated the relationship between the ECL intensity changes of the MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex and various concentrations of Hg2+ and Ag+, respectively. From Fig. 3A could be found that the increased ECL intensities were directly related to the concentration of Hg2+, and the ECL intensity increased linearly with the increased logarithm of the Hg2+ concentration over a range of 10 nM to 150 nM (inset of Fig. 3A). Similarly, the relationship between the ECL intensity and the concentrations of Ag+ over a range of 0.1 nM to 100 nM was also observed in the inset of Fig. 3B. The detection limits of Hg2+ and Ag+ were 10 nM and 0.1 nM, respectively.
On the basis of the restored ECL intensities of the MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex with a wide range of inputs, output was 1 in the presence of Hg2+ (10 nM) or Ag+ (3 nM). Neither Hg2+ (10 nM) nor Ag+ (3 nM) was present, output was 0. The threshold value corresponded to the ECL signal of 10 nM Hg2+ (or 3 nM Ag+), which was in the linear range of the sensor (insets of Fig. 3). Certainly, the inputs “1” and “0” for Hg2+ and Ag+ were also precisely defined. 10 nM was the boundary between input 1 and 0 for Hg2+, while 3 nM was the boundary between input 1 and 0 for Ag+. For example, 0 in the columns of Hg2+ means that it was absent or its concentration was lower than 10 nM.
3.4 INHIBIT gate
Though the OR logic gate is a potential sensor for the detection of multiple analytes, it is difficult to figure out which analyte activates the signal. Two INHIBIT logic gates were designed based on the MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex to promote the application of the MCNTs/aptamer system in complex bioanalysis.The INHIBIT logic gate for Hg2+ was realized by employing Hg2+ as one input, the addition of unlabeled S1 as another input, and the ECL signal was defined as output (Fig. 4A). Ru-silica labeled S1 and S2 were mixed with MCNTs solution to form MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex, in which the ECL intensity of Ru-silica was quenched. Fig. 4B expressed the ECL intensity of MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex at different conditions. The addition of S1 cannot lead to significant ECL enhancement of the MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex while the ECL intensity was largely enhanced with the addition of Hg2+. In the presence of both Hg2+ and S1, T–Hg2+–T complex was formed between Hg2+ and unlabeled S1, thus successfully inhibiting the combination between Hg2+and Ru-silica labeled S1. Consequently, the ECL intensity was scarcely influenced. The results were in accordance with the truth table of the INHIBIT logic gate (Fig. 4C). Correspondingly, Fig. 4D was the symbol of the INHIBIT gate. Subsequently, another INHIBIT logic gate for Ag+ was also designed in the similar way with Ag+ and unlabeled S2 as inputs, which was schematically represented in Fig. S2.†
3.5 NOR gate
In addition, based on the fact that Hg2+ or Ag+ could induce the recovery of the ECL signal of Ru-silica nanoparticles from the MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex, we built two NOR logic gates.First, NOR logic gate for Hg2+ was realized using unlabeled S1 and iodide ions (I−) as inputs, which was shown in Fig. 5A. Fig. 5B showed the ECL intensity of MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex at different conditions. In the gate, Hg2+ could bind with Ru-silica labeled S1 induced the recovery of the ECL intensity of Ru-silica nanoparticles and gave out a high signal when there was no input. Unlabeled S1 and I− could both bind with Hg2+ via forming special structures. Inputting any one or both of them, the Hg2+-induced recovery of the ECL intensity of the Ru-silica was suppressed and output a low signal. The results were in accord with the truth table of the NOR logic gate (Fig. 5C).
Subsequently, another NOR logic gate for Ag+ in the similar way as the previous NOR logic gate was bulit using S2 and cysteine (Cys) as inputs (Fig. S3†). Meanwhile, the analytical performance of I− and cysteine were obtained on the basis of NOR logic gate (Fig. S4†).
3.6 Combinatorial logic gates (OR and INHIBIT)
The combinatorial logic gates (OR and INHIBIT) were employed to make multiplex judgment of what targets were present in the input samples (Fig. 6) according to output results obtained. Correspondingly, the operations and diagnosis were summarized in Table 1. It could be concluded that whether Hg2+ (≥10 nM) or Ag+ (≥3 nM) was present or not logically upon the outputs result.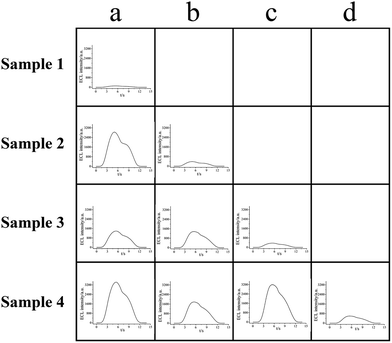 | ||
| Fig. 6 ECL intensity vs. time curves of MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 systems after react with four samples response to the input order in Table 1. Sample 1, no target; sample 2, 50 nM Hg2+; sample 3, 10 nM Ag+; sample 4, 50 nM Hg2+ and 10 nM Ag+. (a–d) Combinatorial logic gates in different kinds (OR and INHIBT). | ||
| Input | Sample | 1 | 1 | 1 | 1 | Diagnosis |
| S1 | 0 | 1 | 0 | 1 | ||
| S2 | 0 | 0 | 1 | 1 | ||
| Output | 0 | \ | \ | \ | No target | |
| 1 | 0 | \ | \ | Contain Hg2+ | ||
| 1 | 1 | 0 | \ | Contain Ag+ | ||
| 1 | 1 | 1 | 0 | Contain Hg2+ and Ag+ | ||
As shown in Fig. 6, identical quantity MCNTs/Ru-silica labeled S1/Ru-silica labeled S2 complex was firstly added to each cell before the addition of samples. The sample 1 (no target) was input into a1 (input 100, the first column in Table 1) under the similar condition used in the OR logic gate. No ECL was obtained (output 0), implying that there was “no target” that neither Hg2+ (≥10 nM) nor Ag+ (≥3 nM) was present. Subsequently there was no need to do other tests (“\” was the symbol of these conditions in Table 1). Similarly, sample 2 was input into a2 cell (input 100, the first column in Table 1) with high ECL intensity obtained (output 1). With the aim of figuring out which target was attributed to the restoration of ECL, the INHIBIT logic gate was subsequently utilized. When S1 and sample 2 was simultaneously input into the system (input 110, the second column in Table 1), there was no apparent ECL intensity observed (output 0), which was attributed to that the input Hg2+ was bound to the other input S1 similar to the condition on the INHIBIT logic gate. Thus it could be deduced that there was Hg2+ (≥10 nM) but not Ag+ (≥3 nM) that existed in sample 2. Correspondingly other tests could be neglected. Sample 3 was input into a3, b3, and c3 cells, in which a3 and b3 showed high ECL intensity (output 1) while only slight ECL signal (output 0) was observed in c3. As S2 and sample 3 were simultaneously input into c3 (input 101, the third column in Table 1), such result indicated that it was Ag+ (≥3 nM) but not Hg2+ (≥10 nM) which induced the high ECL intensity in the input state 100 and 110. Finally, when sample 4 was input into a4, b4, c4, and d4 cells, high ECL signal was obtained in a4, b4, and c4 cells while d4 exhibited a low ECL signal (input 111, the fourth column in Table 1, output 0), manifesting two targets were both bound to their aptamers, respectively. As a result, Hg2+ (≥10 nM) and Ag+ (≥3 nM) were concluded to coexist in sample 4. Meanwhile, it must be noted that either the two targets were absent simultaneously or under their detection limit at the same time, were both possible when the output was diagnosed as “0”. Based on the above analysis, it could be obviously seen that multiplexed ECL systems based on combinatorial logic gates were powerful for the detection of multiple analytes. By such manner, the samples with different targets could be not only analyzed at the same time (as long as aptamers were sufficient) but also diagnosed with assistance from combinatorial logic gates. And thus, it could be regarded to have potentially important applications in DNA logic gates, biosensors, and bioimaging.
3.7 Analytical application potential in lake water
The OR gate was taken as an example to demonstrate analytical application potential of the logic system. As mentioned in Fig. S1,† the logic system was impervious to interference from other metal ions, and the logic system also performed well in practical samples. Fig. 7 showed the response of the logic system upon reaction with the blank lake water and a lake water sample spiked with 50 nM Hg2+ and 10 nM Ag+, which demonstrated that the interference of materials in river water, such as bacteria and pathogenic, could not interact with the probes to double-stranded structures.These logic gates are readily reusable, because of the probe components in the logic devices are adsorbed onto the sensor surface. The signal can regeneration by removing the Hg2+ and Ag+ from the metal–DNA coordinations.44,45 We take the OR gate as example to demonstrate the recovery experiments of the logic gates. The OR gate was recovered in HEPES solution containing 9.0 × 10−4 M cysteine (Cys) allowing interaction for 1 h to remove Hg2+ and Ag+ from the T–Hg2+–T and C–Ag+–C base pairs. The recovery experiments showed acceptable data of the samples with recoveries between 91.2% and 108.9% for the Hg2+, 92.1% and 108.3% for Ag+, while the standard solutions recoveries were between 93.1% and 106.7% for the Hg2+, 93.8% and 106.5% for Ag+. Therefore, the experimental results indicated good accuracy of the proposed logic gate for sample detection in lake water.
4 Conclusions
In summary, the MCNTs/aptamers system was employed to construct a series of two-input DNA logic gates based on the unique interaction between MCNTs and aptamer as well as the utilization of T–T and C–C mismatched probes. It has been demonstrated that the ECL of Ru-silica/TPA system could be quenched by MCNTs via energy-transfer and electron-transfer process. MCNTs are promising electrode materials for high-power and high-energy electrochemical devices. Multiple targets regulated the ECL signals, and different kinds of logic gates were operated by the ECL intensities upon different inputs. Furthermore, OR and INHIBT logic gates were combined to be combinatorial logic gates, which enabled multiplex diagnosis by ECL signal. The proposed logic gates could not only provide a new strategy for the potential application for the monitoring of Hg2+ and Ag+ in environmental samples, but also establish a new approach for multiplex analysis.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (21505052); The Shandong Distinguished Middle-Aged and Young Scientist Encourage and Reward Foundation (BS2014SW035); The Natural Science Foundation of Shandong Province of China (ZR2014BM036); Special Fund for Shandong Independent Innovation and Achievements Transformation (2014ZZCX02703).Notes and references
- A. P. de Silva and N. D. McClenaghan, Chem.–Eur. J., 2004, 10, 574–586 CrossRef PubMed.
- F. Xia, X. Zuo, R. Yang, R. J. White, Y. Xiao, D. Kang, X. Gong, A. A. Lubin, A. Vallee-Belisle, J. D. Yuen, B. Y. Hsu and K. W. Plaxco, J. Am. Chem. Soc., 2010, 132, 8557–8559 CrossRef CAS PubMed.
- E. Katz and V. Privman, Chem. Soc. Rev., 2010, 39, 1835–1857 RSC.
- M. Mayhew, A. C. da Silva, J. Martin, H. Erdjument-Bromage, P. Tempst and F. U. Hartl, Nature, 1996, 379, 420–426 CrossRef CAS PubMed.
- Q. Ouyang, P. D. Kaplan, S. Liu and A. Libchaber, Science, 1997, 278, 446–449 CrossRef CAS PubMed.
- K. Szacilowski, Chem. Rev., 2008, 108, 3481–3548 CrossRef CAS PubMed.
- B. M. Frezza, S. L. Cockroft and M. R. Ghadiri, J. Am. Chem. Soc., 2007, 129, 14875–14879 CrossRef CAS PubMed.
- A. P. de Silva, D. B. Fox, T. S. Moody and S. M. Weir, Trends Biotechnol., 2001, 19, 29–34 CrossRef CAS PubMed.
- R. Freeman, T. Finder and I. Willner, Angew. Chem., Int. Ed., 2009, 48, 7818–7821 CrossRef CAS PubMed.
- J. Ren, J. Wang, J. Wang and E. Wang, Chemistry, 2013, 19, 479–483 CrossRef CAS PubMed.
- J. Zhu, L. Zhang, T. Li, S. Dong and E. Wang, Adv. Mater., 2013, 25, 2440–2444 CrossRef CAS PubMed.
- X. Liu, R. Aizen, R. Freeman, O. Yehezkeli and I. Willner, ACS Nano, 2012, 6, 3553–3563 CrossRef CAS PubMed.
- R. Orbach, F. Remacle, R. D. Levine and I. Willner, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 21228–21233 CrossRef CAS PubMed.
- J. Elbaz, O. Lioubashevski, F. Wang, F. Remacle, R. D. Levine and I. Willner, Nat. Nanotechnol., 2010, 5, 417–422 CrossRef CAS PubMed.
- I. Willner, B. Shlyahovsky, M. Zayats and B. Willner, Chem. Soc. Rev., 2008, 37, 1153–1165 RSC.
- T. Li, E. Wang and S. Dong, J. Am. Chem. Soc., 2009, 131, 15082–15083 CrossRef CAS PubMed.
- B. Y. Chang, J. A. Crooks, K. F. Chow, F. Mavre and R. M. Crooks, J. Am. Chem. Soc., 2010, 132, 15404–15409 CrossRef CAS PubMed.
- X. Li, L. Sun and T. Ding, Biosens. Bioelectron., 2011, 26, 3570–3576 CrossRef CAS PubMed.
- X. Liu and H. Ju, Anal. Chem., 2008, 80, 5377–5382 CrossRef CAS PubMed.
- E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS PubMed.
- H. T. Ratte, Environ. Toxicol. Chem., 1999, 18, 89–108 CrossRef CAS.
- I. Hoyle and R. D. Handy, Aquat. Toxicol., 2005, 72, 147–159 CrossRef CAS PubMed.
- P. Holmes, K. A. James and L. S. Levy, Sci. Total Environ., 2009, 408, 171–182 CrossRef CAS PubMed.
- Y. Miyake, H. Togashi, M. Tashiro, H. Yamaguchi, S. Oda, M. Kudo, Y. Tanaka, Y. Kondo, R. Sawa, T. Fujimoto, T. Machinami and A. Ono, J. Am. Chem. Soc., 2006, 128, 2172–2173 CrossRef CAS PubMed.
- H. Li, J. Zhai and X. Sun, Langmuir, 2011, 27, 4305–4308 CrossRef CAS PubMed.
- G. Zhu, Y. Li and C. Y. Zhang, Chem. Commun., 2014, 50, 572–574 RSC.
- Y. Shan, J. J. Xu and H. Y. Chen, Chem. Commun., 2009, 905–907, 10.1039/b821049g.
- L. Zheng, Y. Chi, Y. Dong, L. Zhang and G. Chen, J. Phys. Chem. C, 2008, 112, 15570–15575 CAS.
- Z. Chen and Y. Zu, J. Phys. Chem. C, 2008, 112, 16663–16667 CAS.
- W. Cao, J. P. Ferrance, J. Demas and J. P. Landers, J. Am. Chem. Soc., 2006, 128, 7572–7578 CrossRef CAS PubMed.
- L. Chen, Q. Cai, F. Luo, X. Chen, X. Zhu, B. Qiu, Z. Lin and G. Chen, Chem. Commun., 2010, 46, 7751–7753 RSC.
- H. Zheng and Y. Zu, J. Phys. Chem. B, 2005, 109, 16047–16051 CrossRef CAS PubMed.
- J. McCall, C. Alexander and M. M. Richter, Anal. Chem., 1999, 71, 2523–2527 CrossRef CAS PubMed.
- H. Li, M. E. Kose, L. Qu, Y. Lin, R. B. Martin, B. Zhou, B. A. Harruff, L. F. Allard and Y.-P. Sun, J. Photochem. Photobiol., A, 2007, 185, 94–100 CrossRef CAS.
- E. Shafran, B. D. Mangum and J. M. Gerton, Nano Lett., 2010, 10, 4049–4054 CrossRef CAS PubMed.
- P. J. Goutam, D. K. Singh and P. K. Iyer, J. Phys. Chem. C, 2012, 116, 8196–8201 CAS.
- S. C. Tsang, Y. K. Chen, P. J. F. Harris and M. L. H. Green, Nature, 1994, 372, 159–162 CrossRef CAS.
- J. Liu, A. G. Rinzler, H. Dai, J. H. Hafner, R. K. Bradley, P. J. Boul, A. Lu, T. Iverson, K. Shelimov, C. B. Huffman, F. Rodriguez-Macias, Y. S. Shon, T. R. Lee, D. T. Colbert and R. E. Smalley, Science, 1998, 280, 1253–1256 CrossRef CAS PubMed.
- X. Tang, D. Zhao, J. He, F. Li, J. Peng and M. Zhang, Anal. Chem., 2013, 85, 1711–1718 CrossRef CAS PubMed.
- M. Li, H. Yang, C. Ma, Y. Zhang, S. Ge, J. Yu and M. Yan, Sens. Actuators, B, 2014, 191, 377–383 CrossRef CAS.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- G. K. Darbha, A. K. Singh, U. S. Rai, E. Yu, H. Yu and P. Chandra Ray, J. Am. Chem. Soc., 2008, 130, 8038–8043 CrossRef CAS PubMed.
- G. H. Clever, C. Kaul and T. Carell, Angew. Chem., Int. Ed., 2007, 46, 6226–6236 CrossRef CAS PubMed.
- J.-S. Lee, P. A. Ulmann, M. S. Han and C. A. Mirkin, Nano Lett., 2008, 8, 529–533 CrossRef CAS PubMed.
- T. Li, L. Shi, E. Wang and S. Dong, Chem.–Eur. J., 2009, 15, 3347–3350 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02531e |
| This journal is © The Royal Society of Chemistry 2016 |