Synthesis of a low-density biopolymeric chitosan–agarose cryomatrix and its surface functionalization with bio-transformed melanin for the enhanced recovery of uranium(VI) from aqueous subsurfaces†
Anuj Tripathi and
Jose Savio Melo*
Nuclear Agriculture and Biotechnology Division, Bhabha Atomic Research Centre, Mumbai-400085, India. E-mail: anujtri@barc.gov.in; jsmelo@barc.gov.in; Fax: +91-22-25505151; Tel: +91-22-25592760
First published on 30th March 2016
Abstract
This study presents the development of a biopolymeric cryomatrix for the recovery of uranium from aqueous subsurfaces. The agarose–chitosan (AC) cryomatrix was synthesized by the process of cryotropic-polymerization at −20 °C. The surface of the AC cryomatrix was further modified by green-chemistry using sequential conversion of L-Dopa to melanin through the biocatalytic activity of tyrosinase that was extracted from the corm of plant tuber Amorphophallus campanulatus. Functionalization of the AC cryomatrix with melanin (MAC cryomatrix) presented enhanced uranium uptake, resistance to degradation along with high thermal and mechanical stability. This cryomatrix showed high interconnected porosity (∼90%), high swelling kinetics and permeability. The optimized ratio of biopolymers and cryogenic parameters led to formation of a buoyant low-density matrix that is favorable for radionuclide recovery from subsurface of contaminated water. Maximum uranium adsorption of the melanin-functionalized agarose–chitosan (MAC) cryomatrix was 97% ± 2% observed at pH 5.5 and the qmax was 435 mg g−1. The thermodynamic parameters suggest passive endothermic adsorption behavior. Furthermore, changes in the rheological properties after uranium binding were studied by micro-rheometry. The binding of uranium and chemical attraction in these cryomatrices was confirmed by FTIR. HCl was found to be an efficient eluent for uranium desorption (92 ± 4%) from the MAC cryomatrix. Five repeated cycles of adsorption–desorption showed the reusability of the MAC cryomatrix. These results suggest stability of the low-density MAC cryomatrix under environmental conditions with the potential for recovery of uranium from contaminated aqueous subsurfaces, including seawater.
1. Introduction
Radionuclide contamination could occur from natural sources, mining-related activities and discharge of low-level nuclear waste into the ocean. A previous study suggests that the marine environment contains sustainable amounts of radionuclides, wherein one cubic meter of seawater typically contains 40 Bq of 238U.1 Recent statistics indicate that approximately 4.58 billion tons of uranium is present in the well-mixed upper surface layer of the oceans.2 From this, around 2 billion tons is considered as accessible for recovery. Additionally, approximately 9000 tons of uranium is also contributed every year by fresh water streams.2 Therefore, the removal of scarce, expensive and biologically hazardous uranium from aqueous subsurfaces is a strong motivation and challenge for the development of a floating sorbent.Considering the requirement for treatment of nuclear wastes, a wide range of different separation approaches like solvent extraction, ion exchange, complexation and precipitation have been developed in the past.3 The process of adsorption by biomaterials is considered as an effective and environmentally friendly approach for the removal of radionuclides from aqueous environments. The adsorption capacity of several low-cost biosorbents, mainly microorganisms or biowaste materials, have been investigated in the past due to the presence of functional chelating moieties on their interfacial surfaces that have shown binding affinity for uranium.4–8 Some of the natural polymers like alginate, agarose and chitosan, which are mainly derived from seaweed and shells of shrimp, respectively, are abundantly available in nature, but have not shown high sorption efficacy due to an unsuitable way of deployment. In order to overcome the limitations associated with this, one of our recent studies successfully demonstrated that uranium present on the upper subsurface layer of seawater can be recovered by rapid sorption kinetics using novel macroporous cryobead formulations that have chelating moieties from alginate polymer.9 However, the fast degradation, microbial susceptibility, non-specific metal affinity, and low sorption capacity were major limitations with these cryobead formulations.
Natural pigments, like melanin, have also been recently found to be a potential metal sorbent.10 However, due to its limited availability in nature and difficulty of isolation in large quantities, thereby making its cost prohibitive, commercial applications of melanin are limited. On the other hand, melanin that is synthesized by a chemical or biological route is obtained as macro-granules and shows poor diffusion properties, thus requiring higher sorption times. Besides, the high density of melanin granules also does not permit its application for uranium sorption from aqueous subsurfaces. These drawbacks need to be addressed in order to exploit its potential in uranium recovery from subsurface seawater.
The current state of material research encourages developing porous biomaterials with improved surface sorption capacities and floating properties combined with resistance to biodegradation.9 Low-temperature polymerization, i.e. cryopolymerization of the precursor units of a polymer or mixture of polymers, is a emerging approach showing potential for the development of macroporous polymeric matrices for different bioengineering applications.11–13 These gels comprise macroporous interconnected pore morphologies with appropriate mechanical integrity, which is advantageous over classical hydrogels.14 Moreover, the designing of porous matrices using biopolymers in the bead form provide a large surface area for increasing the binding of metals.9 Thus, we propose to develop an integrated process that involves the synthesis of a novel, low-density, floating bio-polymeric matrix and further surface functionalize with bio-melanin for enhanced recovery of uranium from aqueous environments. Thus, this study aims to develop a feasible formulation in the form of a porous cryomatrix using non-toxic biopolymers like agarose and chitosan. In addition, process development involved the optimization of in situ functionalization of the cryomatrix with bio-melanin. The physical, chemical and rheological properties of the matrix were examined. Finally, the cryomatrix was used for uranium sorption and the sorption kinetics, effect of pH, concentration, temperature and cyclic adsorption–desorption potential using a simulated aqueous environment was examined.
2. Materials and methods
2.1. Preparation of agarose–chitosan (AC) cryomatrix
Chitosan powder (MW: 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–190
000–190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da; degree of deacetylation ≥75% and viscosity: 20–300 cps; Sigma-Aldrich, Germany; 100 mg) was dissolved in 5 mL of 1% acetic acid solution (pH ∼ 2.5) with the help of mechanical stirring at room temperature. In a separate tube, agarose low EEO (–Mr: 0.09–0.13, gelling strength: ∼1200 g cm−2, gelling temperature: ∼38–40 °C; SRL, India; 300 mg) was dissolved in 5 mL of deionized water (dH2O) by boiling. Once a transparent solution of agarose was obtained, the chitosan solution was added to it. This polymer mixture was further kept at room temperature (25 ± 2 °C) until the solution temperature dropped to ∼45 °C. Glutaraldehyde solution (80 μL of 25%, v/v dissolved in 420 μL of dH2O; final concentration 0.2%, v/v; Sigma-Aldrich, Germany) was then added to the polymer mixture and was gently mixed to avoid bubbles. This mixture was immediately transferred to a 10 mL plastic syringe (Becton Dickinson, India) connected with a hypodermic needle (18 gauge; Star Enterprises, India). The polymer mixture was dropped into moderately frozen liquid paraffin oil (light viscous; Loba Chemie, India) and incubated at −20 °C for 16 h. After incubation, agarose–chitosan (AC) polymeric beads were sieved from the paraffin oil and repeatedly washed with dH2O to remove the paraffin oil. The composite cryomatrix of agarose and chitosan thus synthesized were air-dried at room temperature for further studies.
000 Da; degree of deacetylation ≥75% and viscosity: 20–300 cps; Sigma-Aldrich, Germany; 100 mg) was dissolved in 5 mL of 1% acetic acid solution (pH ∼ 2.5) with the help of mechanical stirring at room temperature. In a separate tube, agarose low EEO (–Mr: 0.09–0.13, gelling strength: ∼1200 g cm−2, gelling temperature: ∼38–40 °C; SRL, India; 300 mg) was dissolved in 5 mL of deionized water (dH2O) by boiling. Once a transparent solution of agarose was obtained, the chitosan solution was added to it. This polymer mixture was further kept at room temperature (25 ± 2 °C) until the solution temperature dropped to ∼45 °C. Glutaraldehyde solution (80 μL of 25%, v/v dissolved in 420 μL of dH2O; final concentration 0.2%, v/v; Sigma-Aldrich, Germany) was then added to the polymer mixture and was gently mixed to avoid bubbles. This mixture was immediately transferred to a 10 mL plastic syringe (Becton Dickinson, India) connected with a hypodermic needle (18 gauge; Star Enterprises, India). The polymer mixture was dropped into moderately frozen liquid paraffin oil (light viscous; Loba Chemie, India) and incubated at −20 °C for 16 h. After incubation, agarose–chitosan (AC) polymeric beads were sieved from the paraffin oil and repeatedly washed with dH2O to remove the paraffin oil. The composite cryomatrix of agarose and chitosan thus synthesized were air-dried at room temperature for further studies.
2.2. Functionalization of AC cryomatrix with melanin
The porous agarose–chitosan (AC) cryomatrix was functionalized with melanin by in situ biocatalysis of 3,4-dihydroxyphenylalanine (L-Dopa; ≥98%, Sigma-Aldrich, Germany) using tyrosinase enzyme. Crude tyrosinase enzyme used in this study was partially extracted from corm of the tuber of Amorphophallus campanulatus as per the procedure described in our previous study.15 For functionalization, AC cryomatrix (100 mg) was added to 20 mL of freshly prepared L-Dopa solution (10 mM) and incubated for 30 min at 37 °C, which allows possible physical adsorption of L-Dopa onto the matrix surface. Then, 100 μL of tyrosinase enzyme (enzyme activity 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1 in 0.1 M phosphate buffer, pH 6) was added to this solution and mixed using a mechanical rocker (Bangalore Genei, India) at 15 rpm for 24 h in the dark at room temperature. After completion of the reaction, the melanin-functionalized agarose–chitosan (MAC) matrix was subjected to thorough washing using deionized water to remove any unreacted substrate and free-enzyme. This matrix was air-dried and stored in an airtight tube at room temperature for further characterization. For reproducibility, the experiment was repeated five different times under the same conditions.
000 U mL−1 in 0.1 M phosphate buffer, pH 6) was added to this solution and mixed using a mechanical rocker (Bangalore Genei, India) at 15 rpm for 24 h in the dark at room temperature. After completion of the reaction, the melanin-functionalized agarose–chitosan (MAC) matrix was subjected to thorough washing using deionized water to remove any unreacted substrate and free-enzyme. This matrix was air-dried and stored in an airtight tube at room temperature for further characterization. For reproducibility, the experiment was repeated five different times under the same conditions.
2.3. Physicochemical characterization of AC and MAC cryomatrices
AC and MAC cryomatrices were examined for various physicochemical properties like porosity and internal architecture, swelling kinetics and swelling ratio, hydraulic permeability, density, degradation resistance, zeta potential, thermogravimetric behavior, chemical attribution of functional groups by FTIR and also rheological behavior. The details of the methodology are described in the ESI data.†2.4. Study of uranium sorption
The sorption experiments were performed using 25 mg of AC and MAC cryomatrices (dimensions ∼2 mm) suspended in 25 mL of uranium solution having an initial concentration of 100 mg L−1 at the agitation speed of 25 rpm at different pH's and temperatures. After the predefined incubation time, the samples were collected for quantitative analysis of remaining uranium in the aqueous medium. The uranium solution without the test sample was used as a control for each set of experiments. The quantification of uranium concentration in the solution was performed using the Arsenazo (III) method16 (method described in ESI data†).The adsorption equilibrium of uranium per unit of sample, i.e. mg of uranium per gram dry weight of sample, was determined using eqn (1):
| qe = (Co − Ce)(V/M) | (1) |
However, the distribution coefficient (Kd) (mL g−1) was obtained using eqn (2):
| Kd = [(Co − Ce)/Ce](V/M) | (2) |
| Ad (%) = [(Co − Ce)/Co] × 100 | (3) |
| Ce/qe = [(1/qmax)(1/b)] + Ce/qmax | (4) |
Linearised form of the Freundlich isotherm was used as given in eqn (5);
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf + 1/n(ln Kf + 1/n(ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce) Ce)
| (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe vs. ln
qe vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce and the slope and intercept were used to calculate the Freundlich parameters.
Ce and the slope and intercept were used to calculate the Freundlich parameters.
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd = (ΔS°/R) − (ΔH°/RT) Kd = (ΔS°/R) − (ΔH°/RT)
| (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd against 1/T using a least-squares curve-fitting program. Furthermore, the enthalpy and entropy values were used to calculate the Gibbs free energy (ΔG) using eqn (7):
Kd against 1/T using a least-squares curve-fitting program. Furthermore, the enthalpy and entropy values were used to calculate the Gibbs free energy (ΔG) using eqn (7):| ΔG = ΔH − TΔS | (7) |
The qe desorption (mg g−1) of uranium was calculated directly from the amount of uranium adsorbed and amount of uranium desorbed using eqn (8):
| qe (desorption) = (Madsorb − Mdesorb) × V/M | (8) |
The percent desorption was obtained from eqn (9):
| Desorption (%) = (Mdesorb/Madsorb) × 100 | (9) |
3. Results and discussion
3.1. Synthesis and functionalization of the AC cryomatrix
The AC cryomatrix was synthesized by the water-in-oil emulsion procedure at cryogenic temperatures under static conditions (see ESI data† for cryo-polymerization effect). An interconnected porous network is obtained in the AC cryomatrix after thawing of these matrices in dH2O. During the process of cryo-polymerization, the inter and intra chain crosslinking between reactive functional groups of agarose and chitosan was governed by glutaraldehyde, which crosslinks the amine groups of chitosan and the hydroxyl groups of agarose (Fig. 1).11,21,22 The optimized 4% concentration in the cryomatrix has a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of agarose to chitosan. These cryomatrices were approximately 2 mm in diameter. The composite cryomatrix of AC and MAC retain their shape after drying at room temperature. The cryomatrix exhibited opaque and spongy-like properties contrary to its hydrobead counterpart that was synthesized at room temperature and was semi-transparent, non-spongy and light yellow in color. Moreover, the hydrobead lost its original dimensions when dried at room temperature, which is a typical behavior for that hydrogel (ESI data Fig. S1†).
1 ratio of agarose to chitosan. These cryomatrices were approximately 2 mm in diameter. The composite cryomatrix of AC and MAC retain their shape after drying at room temperature. The cryomatrix exhibited opaque and spongy-like properties contrary to its hydrobead counterpart that was synthesized at room temperature and was semi-transparent, non-spongy and light yellow in color. Moreover, the hydrobead lost its original dimensions when dried at room temperature, which is a typical behavior for that hydrogel (ESI data Fig. S1†).
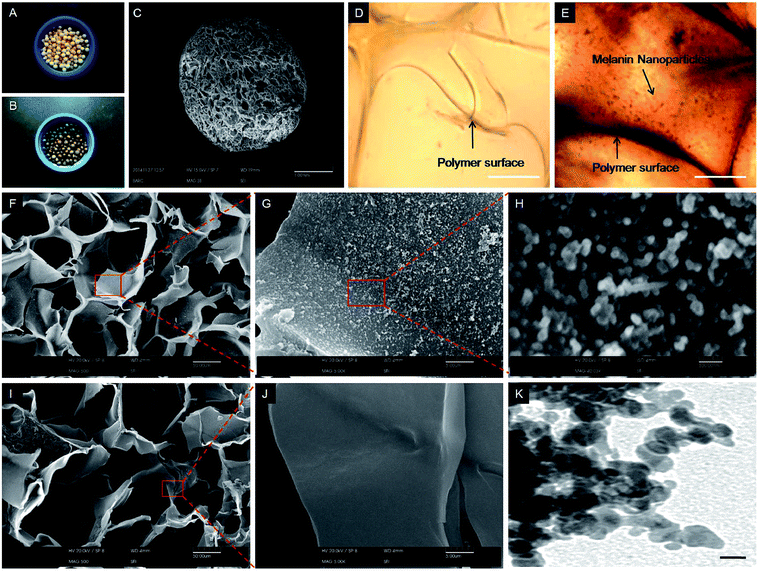
Functionalization of the AC cryomatrix with melanin was optimized by observing the change in color from pale yellow to blackish-brown (Fig. 2A and B). The matrix showed a uniform pore distribution (Fig. 2C). Before and after melanin functionalization, the matrices were examined using differential interference contrast (DIC) microscopy and SEM. The DIC microscopy analysis confirmed the presence of melanin nanoparticles on the surface of the cryomatrix after functionalization (Fig. 2D and E). The SEM analysis further confirmed the uniform distribution of melanin nanoparticles on the surface of the MAC cryomatrix (Fig. 2F–H). Besides, the AC matrix showed a smooth surface property (Fig. 2I and J). The SEM analysis also showed the spherical morphology of melanin nanoparticles that were in the size range of ∼100 nm, and this was further validated by TEM analysis (Fig. 2K). For 100 mg AC cryomatrix, the optimum concentration of L-DOPA was 10 mM in 20 mL solution. The percent conversion of L-DOPA to melanin was estimated to be 37.5%, which was deposited on the surface of the cryomatrix. The gravimetric analysis of dry AC and MAC samples suggest the aspect ratio of the matrix to melanin was 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
3.
3.2. Physicochemical properties of AC and MAC cryomatrices
Scanning electron micrographs showed the presence of a porous internal architecture in the MAC cryomatrix (Fig. 2C). The pores were in the micron-size range, were interconnected and were uniformly distributed (Fig. 2D). The uniform deposition of melanin on these matrices was observed from the periphery to the core of the matrix. The physicochemical properties of the MAC cryomatrix are shown in Table 1.| Properties | Agarose–chitosan (AC) cryomatrix | Melanin–agarose–chitosan (MAC) cryomatrix | |
|---|---|---|---|
| Porosity | ∼90% | ∼89% | |
| Average pore size | 40–90 μm | 40–90 μm | |
| Swelling ratio | 15.68 ± 0.44 | 13.72 ± 0.53 | |
| Swelling equilibrium time | ∼2.5 min | ∼2.5 min | |
| Hydraulic permeability | 7.9 × 10−4 m4 N−1 s−1 | 7.2 × 10−4 m4 N−1 s−1 | |
| Flow rate | Up to 10 mL m−1 | Up to 10 mL m−1 | |
| Density | Dry | 0.054 ± 0.001 g cm−3 | 0.064 ± 0.003 g cm−3 |
| Wet | 0.838 ± 0.019 g cm−3 | 0.890 ± 0.019 g cm−3 | |
| Degradation (in four weeks) | 19.56 ± 2.91% | 8.21 ± 1.26% | |
| Thermal stability | Up to 210 °C | Up to 210 °C | |
| Zeta potential (at pH 5.5) | + 2.3 ± 0.34 mV | −19.2 ± 1.23 mV | |
| Complex modulus | Before U-bound | 4.0 MPa | 4.8 MPa |
| After U-bound | 4.6 MPa | 6.1 MPa | |
The size of the pores was in the range 20 to 210 μm and the average pore diameter was in the range of 40 to 90 μm in the MAC cryomatrix. Similar pore morphologies were observed in the AC cryomatrix. The calculated average porosity of the AC and MAC cryomatrices were approximately 90% and 89%, respectively. Both AC and MAC cryomatrices showed up to 10 mL min−1 of liquid cross-sectional flow without any flow resistance. The calculated hydraulic permeability of the AC and MAC polymer matrices was 7.9 × 10−4 m4 N−1 s−1 and 7.2 × 10−4 m4 N−1 s−1, respectively. These values are suggestive of highly interconnected porosity, in both the cryomatrices, and could be of potential use for applications involving the processing of large volumes of liquids.
The use of hydrophilic polymers, i.e. agarose and chitosan, in a composite form shows high swelling kinetics (rate of solvent uptake with respect to time) in an aqueous system (Fig. 3). Both the cryomatrices swelled up to ∼90% of their capacity within 90 s and reached equilibrium within 2.5 min at room temperature (∼25 °C) (Fig. 3A). The fast water uptake was probably due to the presence of the interconnected pores that act as a capillary network. However, the quick equilibrium is also suggestive of the hydrophilic nature of cryomatrix. The presence of high amounts of carboxylic groups and prevention of agglomeration from the melanin moieties to avoid non-polarity have facilitated the hydrophilic nature in melanin. This is in agreement to the recent study showing the synthesis of water soluble melanin nanoparticles.23 The swelling ratio of the AC and MAC cryomatrices were 15.68 ± 0.44 and 13.72 ± 0.53, respectively (Table 1), which suggests that these cryomatrices have high water retaining capacities.
3.3. Floating behavior of AC and MAC cryomatrices
Application of this low-density floating matrix as a potential sorbent for the recovery of uranium from aqueous subsurfaces is suggested.24,25 Thus, we have synthesized a low-density composite cryomatrix considering the fact that the apparent density of water varies in the range of 0.97 to 1 g cm−3 (seawater: 1.027 g cm−3) depending on the temperature. The dry and wet density of the AC cryomatrix was found to be 0.054 ± 0.001 g cm−3 and 0.838 ± 0.019 g cm−3, respectively. However, the MAC cryomatrix showed an increased density (dry: 0.064 ± 0.003 g cm−3 and wet: 0.890 ± 0.019 g cm−3), but it was not higher than the density of water. Thus, the floating behavior of both AC and MAC cryomatrices (Fig. 3B) is suggested. During the study, it was also observed that the AC and MAC cryomatrices maintained their floating property at different temperatures (15 °C to 45 °C).3.4. Degradation of AC and MAC cryomatrices
Major concerns exist over the biodegradation of natural polymers such as chitosan and agarose during environmental applications.26 Chemical modifications of such materials have been suggested to improve their suitability for marine applications in term of resistance to mechanical stress and decreased biodegradation.27 Degradation of the glutaraldehyde crosslinked AC cryomatrix was 19.56 ± 2.91% after four weeks of incubation in non-sterile water at 37 °C. The MAC cryomatrix showed significantly less degradation (8.21 ± 1.26%) compared to the AC cryomatrix (p < 0.05) (Fig. 3C). The degradation of cryomatrix was reduced probably due to the inter- and intra-chain covalent crosslinking11 of agarose and chitosan, as shown in Fig. 1, that reduces the interaction to water molecules. On the other hand, the backbone of melanin is composed of hydrophobic ring structures28 that probably do not allow the penetration of water molecules. Thus, its deposition on the cryomatrix reduces hydrolytic degradation. Additionally, previous studies support the anti-microbial property of melanin,29,30 which could also be a cause of slow biodegradation of the MAC cryomatrix. The study supports that the functionalization with melanin causes a positive impact on the MAC cryomatrix, which increased its resistance to biodegradation.3.5. Thermal properties of cryomatrices
Thermal stability of the polymer composite was confirmed by TGA (Fig. 4A). The thermo-gravimetric analysis showed ∼8% water loss at around 90 °C in both AC and MAC cryomatrices due to the release of polymer-bound water. The onset of AC and MAC cryomatrices degradation was observed at 210 °C, followed by a sudden decrease in mass. The lower rate of decomposition is attributed to higher intermolecular bonding between the biopolymers. Approximately 50% weight loss of AC and MAC cryomatrices was observed at 316 °C and 432 °C, respectively. However, at the end of the analysis (700 °C), the thermal profile showed 71% polymer decomposition in the AC cryomatrix compared to 57% decomposition in the MAC cryomatrix. These results suggest the higher stability of MAC cryomatrix over AC cryomatrix. The solidus temperature of these cryomatrices was found to be ∼200 °C. These results suggest that AC and MAC cryomatrices can be used over a wide temperature range.3.6. Zeta potential of AC and MAC cryomatrices
The AC cryomatrix showed a zeta potential (ζ) of +10.2 ± 0.81 mV, +2.3 ± 1.23 mV and −18.6 ± 2.46 mV at pH 3, pH 5.5 and pH 11, respectively; and a point of zero charge (PZC) was obtained near pH 5.7 (Fig. 4B). Below the PZC, free amine groups (R–NH2) on chitosan are protonated to the ionic form (R–NH+3) and thus, provide net positive surface charge to the matrix.31 The negative zeta potential above the PZC could be possible because of OH− ions. In the case of the MAC cryomatrix (Fig. 4B), the zeta potential was positive i.e. +4.8 ± 0.87 mV at pH 3, and showed a high negative zeta potential −19.2 ± 1.45 mV at pH 5.5. Furthermore, increases in pH did not show significant changes to the zeta potential and it was found to be −18.4 ± 0.35 mV at pH 11. The PZC for the MAC cryomatrix was observed at around pH 3.4. The MAC cryomatrix is speculated to present abundant hydroxyl and carboxyl groups on the surface, which can form stable interactions with uranyl ions (Fig. 4C). These groups are protonated at lower pH, but exhibit a net negative charge on polymer surfaces. The presence of free amines on chitosan may be suppressed either by partial masking or by interacting with melanin polymer chain, which is a key factor in establishing the PZC for the MAC cryomatrix. The high negative zeta potential of free melanin supports our speculation that the change in surface properties of the AC cryomatrix after its functionalization with melanin is caused by melanin. It is also believed that the high negative surface charge in acidic pH could translate to high affinity to uranyl ions in an aqueous medium.3.7. Uranium sorption study
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H···N)2− (ESI Fig. S2†),36 and this could be a reason for the strong adsorption of UO22+ to the AC cryomatrix. Besides, increasing or decreasing the pH of the solution from pH 5.5 led to decreases in the adsorption of uranium as shown in Fig. 5A. This behavior suggests the possibility of higher concentrations of hydrolyzed ions like H+ and H3O+ in the solution at low pH that may hinder the binding of UO22+ ions to the polymer surface. However, at higher pH, the reason for the decrease in the adsorption of uranium could be because of the formation of carbonate complexes of uranium in the presence of atmospheric CO2 and Na2CO3.37 At a pH below 6 (under normal oxidizing conditions), uranium is allowed to stay in solution as [(UO2)3OH]5+ instead of precipitation as a carbonate.38 In general, at a pH near the pKa value of the functional groups present on the polymer, the highest ionization for binding to uranium is shown. Thus, the carboxyl and hydroxyl group rich MAC cryomatrix showed higher uranium sorption at pH 5.5. Therefore, the pH dependent decrease in sorption was either directly proportional to the decrease in the number of available negative charges on the surface of cryomatrix at different pH, or the decrease in the ionic form of uranium above pH 5.5.
O⋯H···N)2− (ESI Fig. S2†),36 and this could be a reason for the strong adsorption of UO22+ to the AC cryomatrix. Besides, increasing or decreasing the pH of the solution from pH 5.5 led to decreases in the adsorption of uranium as shown in Fig. 5A. This behavior suggests the possibility of higher concentrations of hydrolyzed ions like H+ and H3O+ in the solution at low pH that may hinder the binding of UO22+ ions to the polymer surface. However, at higher pH, the reason for the decrease in the adsorption of uranium could be because of the formation of carbonate complexes of uranium in the presence of atmospheric CO2 and Na2CO3.37 At a pH below 6 (under normal oxidizing conditions), uranium is allowed to stay in solution as [(UO2)3OH]5+ instead of precipitation as a carbonate.38 In general, at a pH near the pKa value of the functional groups present on the polymer, the highest ionization for binding to uranium is shown. Thus, the carboxyl and hydroxyl group rich MAC cryomatrix showed higher uranium sorption at pH 5.5. Therefore, the pH dependent decrease in sorption was either directly proportional to the decrease in the number of available negative charges on the surface of cryomatrix at different pH, or the decrease in the ionic form of uranium above pH 5.5.
Langmuir and Freundlich isotherm models were used to characterize the interaction of uranyl ion to the cryomatrices (AC and MAC) (Fig. 6). The Langmuir isotherm explains the maximum monolayer adsorption capacity of any material having finite ligands for binding. The Freundlich isotherm suggests that the strong binding sites of an adsorbent will be occupied first, resulting in a decrease in the binding energy by increasing the degree of occupation and thus, variation of adsorption at variable temperatures. These isotherms are suitable for defining the maximum sorption capacity of any sorbent using theoretical modeling. Adsorption curve data of the MAC cryomatrix was fitted to a linearised form of both isotherms, which are expressed as Langmuir parameters (b = 0.043 L mg−1, qmax = 435 mg g−1 and r2 = 0.996) (Fig. 6A) and Freundlich parameters (n = 3.22, Kf = 74.81 mg g−1 and r2 = 0.967) (Fig. 6B). The qmax of the MAC cryomatrix was obtained from the slope of the graph plotted between Ce/qe and Ce (Fig. 6A). The MAC cryomatrix showed the maximum adsorption capacity of 435 mg g−1 at a temperature of 35 °C. The Freundlich parameters, i.e. Kf and n, were derived from the intercept and the slope of the graph plotted between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe and ln
qe and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce. The value of n depends upon the distribution of active adsorption sites and adsorption energy. The calculated value of n at 35 °C was 3.22. A value for n between >1 and <10 is suggestive of a favorable adsorption process.39 The value of Kf was 74.81 mg g−1, which suggests the adsorption capacity of the sorbent. The mathematically predicted isotherm parameters suggest the heterogeneity in the surface of the MAC cryomatrix and exponential distribution of functional ligands and their binding energies, which is generally an indication of multilayer sorption behavior for the adsorbent.40
Ce. The value of n depends upon the distribution of active adsorption sites and adsorption energy. The calculated value of n at 35 °C was 3.22. A value for n between >1 and <10 is suggestive of a favorable adsorption process.39 The value of Kf was 74.81 mg g−1, which suggests the adsorption capacity of the sorbent. The mathematically predicted isotherm parameters suggest the heterogeneity in the surface of the MAC cryomatrix and exponential distribution of functional ligands and their binding energies, which is generally an indication of multilayer sorption behavior for the adsorbent.40
However, the r2 value suggested goodness of fit for the Langmuir isotherm, which suggests monolayer sorption of uranium onto the surface of the MAC cryomatrix. Similarly, mathematical modeling of the AC cryomatrix also shows that the best data fitting is achieved with the Langmuir isotherm (parameters: b = 0.004 L mg−1, qmax = 204 mg g−1 and r2 = 0.984) (Fig. 6C) compared to the Freundlich isotherm (parameters: n = 1.64, Kf = 3.69 mg g−1 and r2 = 0.957) (Fig. 6D). These values suggest dominance of monolayer adsorption in the cryomatrix but showed and almost two times higher adsorption capacity of the MAC cryomatrix compared to the AC cryomatrix. Considering the sorption capacity of the AC cryomatrix, it is speculated that the minimum sorption capacity of melanin in the MAC cryomatrix was approximate fifty percent of its maximum uptake. Thus, the present study shows that melanin, in the form of smaller particles, can increase the sorption capacity by three-fold in comparison to the macro-granular melanin reported earlier.10 ESI Table S1† shows the comparative uranium sorption capacities of various previously studied biosorbents.
3.8. Thermodynamic studies of uranium sorption on MAC cryomatrix
The MAC cryomatrix was used for evaluating the effect of temperature on uranium sorption (Fig. 7A). The thermodynamic parameters like enthalpy (ΔH) and entropy (ΔS) were calculated from the graph plotted between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd vs. 1/T (K) values, which were then used to calculate the Gibbs free energy (ΔG). The negative ΔH (−30.5 kJ mol−1) is suggestive of an endothermic nature that favors the adsorption of uranium ions at higher temperatures. The positive ΔS (0.134 kJ mol−1 K−1) is suggestive of higher randomness at the solid–solution interface during adsorption, and favors the stability of the adsorption and confirms that the UO22+ ions were not limited during the adsorption process on the adsorbent. These results suggest complex binding and steric hindrance of the uranyl ions to the polymer surface beside the physical adsorption.41 The resultant change in the ΔG showed negative values of −70.3 kJ mol−1 at 298 K, which suggests the spontaneous and high affinity of U(VI) adsorption to the MAC cryomatrix.
Kd vs. 1/T (K) values, which were then used to calculate the Gibbs free energy (ΔG). The negative ΔH (−30.5 kJ mol−1) is suggestive of an endothermic nature that favors the adsorption of uranium ions at higher temperatures. The positive ΔS (0.134 kJ mol−1 K−1) is suggestive of higher randomness at the solid–solution interface during adsorption, and favors the stability of the adsorption and confirms that the UO22+ ions were not limited during the adsorption process on the adsorbent. These results suggest complex binding and steric hindrance of the uranyl ions to the polymer surface beside the physical adsorption.41 The resultant change in the ΔG showed negative values of −70.3 kJ mol−1 at 298 K, which suggests the spontaneous and high affinity of U(VI) adsorption to the MAC cryomatrix.
3.9. Desorption analysis
Desorption of uranium from the uranium-loaded MAC cryomatrix may permit its reusability and could reduce the time and cost of processing for every fresh batch. In the initial screening, four desorbents (NaOH, Na2CO3, NaNO3 and CaCl2) showed poor recovery (i.e. 10–55%) of bound uranium from the MAC cryomatrix (data not shown). The other four desorbents, H2SO4, HCl, HNO3 and EDTA, showed 79%, 92%, 83% and 89% desorption, respectively. The highest desorption was found in the case of HCl and EDTA. Therefore, to confirm the long term effect of these two eluents on the MAC cryomatrix, incubation of the cryomatrices in the respective aqueous media for 60 days was monitored. A prominent white precipitate was observed while incubating the MAC cryomatrix in EDTA (ESI Fig. S3†). In the other medium, MAC cryomatrix suspended in the HCl solution did not show any changes. Thus, HCl was further used for repetitive sorption–desorption upto five cycles under identical experimental conditions. The MAC cryomatrix showed ∼18% and 14% reduction in the adsorption and desorption capacities, respectively, after five cycles of consecutive use (Fig. 7B). However, uranium recovery remained constant in each cycle. Moreover, the possibility of surface functionalization with melanin after multiple sorption–desorption would also be an advantage to using the same material. These results show the potential reusability of the MAC cryomatrix for recovery of uranium from an aqueous medium.3.10. Uranium sorption from seawater
The results of uranium adsorption from natural seawater (NSW) and artificial seawater (ASW) are shown in Fig. 7C. In this study, besides the seawater effect, the effect of sorbent concentration (i.e. 1 g L−1 and 0.25 g L−1) was also monitored for uranium sorption. The MAC cryomatrix at a 1 g L−1 concentration showed 92 ± 1.5% and 86 ± 2% of uranium sorption from ASW and NSW, respectively. However, the sorption capacity of the cryomatrix in ASW and NSW was decreased to 78 ± 2% and 63 ± 3%, respectively, after reducing the initial mass of the MAC cryomatrix four-fold. The sorption was not found directly proportional to the sorbent concentration. The floating MAC cryomatrix are less effective on uranium binding in the presence of sea salts, i.e. 5% reduction in sorption capacity, compared to deionized water and thus, this could be an area of interest for future studies on radionuclides recovery from sea subsurfaces.3.11. Rheological properties of cryomatrices before and after uranium sorption
The rheological behaviour of dry AC and MAC cryomatrices before and after uranium sorption was interpreted from the graph plotted between complex modulus (G*) and variable forces applied at increasing time (Fig. 7D). The G* of the AC cryomatrix was 4 MPa at 5 N, which was increased to 4.6 MPa after uranium adsorption (Table 1). However, the MAC cryomatrix showed a higher G* (4.8 MPa) compared to the AC cryomatrix, which could be because of melanin. After uranium adsorption, the G* of the MAC cryomatrix increased to ∼6.1 MPa (Table 1). The relative increase in G* of the AC cryomatrix after uranium sorption was not higher than the MAC cryomatrix, which is in agreement with the qmax of the AC and MAC cryomatrices. Above normal force of 5 N, these cryomatrices showed deformation that resulted in a steep decrease in the G* (Fig. 7D). These rheological properties suggest that binding of uranium to the cryomatrices increases its mechanical stiffness when subjected to uranium sorption and were stable up to 5 N of force.3.12. FT-IR spectroscopy of cryomatrices before and after uranium sorption
The conformational changes in the functional groups of composite AC and MAC cryomatrices were examined by Fourier transform infrared (FTIR) spectroscopy before and after uranium sorption (Fig. 7E). The basic characteristic peaks of the polymers are explained in the ESI data.† Besides the corresponding characteristic peaks, an absorption band at 1660 cm−1 is attributed to the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds in the composite AC and MAC cryomatrices by glutaraldehyde crosslinking.42 However, a slight band shift at 1650 cm−1 was monitored after uranium sorption, which possibly suggest the stretching of these groups during uranium binding. A broad band at 3200–3500 cm−1 is attributed to the stretching vibrations of –OH and –NH2 in the AC and MAC cryomatrices. However, this band width reduced after uranium sorption and suggests an interaction of these functional groups to uranyl ions. After melanin deposition on the AC cryomatrix, the asymmetric CH2 peak (2950 cm−1) broadened, probably due to the intermolecular complexation and random polymerization of melanin on the cryomatrix surface. Moreover, the intensity of the asymmetric peak was higher compared to the symmetric alkyl peak after uranium sorption, which could be because of molecular bending in the polymer chain for formation of the uranium interaction. The energy band at 933 cm−1 is related to the stretching frequency of the linear O
N bonds in the composite AC and MAC cryomatrices by glutaraldehyde crosslinking.42 However, a slight band shift at 1650 cm−1 was monitored after uranium sorption, which possibly suggest the stretching of these groups during uranium binding. A broad band at 3200–3500 cm−1 is attributed to the stretching vibrations of –OH and –NH2 in the AC and MAC cryomatrices. However, this band width reduced after uranium sorption and suggests an interaction of these functional groups to uranyl ions. After melanin deposition on the AC cryomatrix, the asymmetric CH2 peak (2950 cm−1) broadened, probably due to the intermolecular complexation and random polymerization of melanin on the cryomatrix surface. Moreover, the intensity of the asymmetric peak was higher compared to the symmetric alkyl peak after uranium sorption, which could be because of molecular bending in the polymer chain for formation of the uranium interaction. The energy band at 933 cm−1 is related to the stretching frequency of the linear O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (range 928–936 cm−1) structure in AC-U and MAC-U cryomatrices. Hence, the binding mechanism can be considered as a complexation process between the U(VI) ions and carboxyl, hydroxyl or phenolic groups. However, the lower peak intensity in the AC-U cryomatrix confirms lower uranium sorption in comparison to the MAC-U cryomatrix. The peak at 1163 cm−1 corresponds to C–O stretching of alcohols and carboxylic groups, which was monitored in both AC and MAC cryomatrices. Broadening of this peak after uranium sorption in AC-U and MAC-U cryomatrices also confirmed the involvement of hydroxyl and carboxyl moieties in uranium sorption. One prominent peak at 1530 cm−1 for N–O asymmetric stretching was observed in both uranium bound cryomatrices (AC-U and MAC-U) and is suggestive of nitrogen and oxygen coordination during uranium sorption. Another peak at 1370 cm−1 represents the rocking vibration of C–H bonds observed in MAC, AC-U and MAC-U, but was absent in AC cryomatrix. This rocking vibration is either because of conformational changes in the reactive groups during uranium sorption or because of melanin-functionalization.
O (range 928–936 cm−1) structure in AC-U and MAC-U cryomatrices. Hence, the binding mechanism can be considered as a complexation process between the U(VI) ions and carboxyl, hydroxyl or phenolic groups. However, the lower peak intensity in the AC-U cryomatrix confirms lower uranium sorption in comparison to the MAC-U cryomatrix. The peak at 1163 cm−1 corresponds to C–O stretching of alcohols and carboxylic groups, which was monitored in both AC and MAC cryomatrices. Broadening of this peak after uranium sorption in AC-U and MAC-U cryomatrices also confirmed the involvement of hydroxyl and carboxyl moieties in uranium sorption. One prominent peak at 1530 cm−1 for N–O asymmetric stretching was observed in both uranium bound cryomatrices (AC-U and MAC-U) and is suggestive of nitrogen and oxygen coordination during uranium sorption. Another peak at 1370 cm−1 represents the rocking vibration of C–H bonds observed in MAC, AC-U and MAC-U, but was absent in AC cryomatrix. This rocking vibration is either because of conformational changes in the reactive groups during uranium sorption or because of melanin-functionalization.
4. Conclusion
The present study demonstrates the fabrication of a porous low-density cryomatrix using cryo-polymerization. This one-step process of designing the porous system using polymeric precursors in the form of a bead is attractive. Furthermore, crude plant-derived extract containing tyrosinase was used for in situ biogenic transformation of L-Dopa into melanin on the cryomatrix surface. The melanin-functionalized agarose–chitosan cryomatrix showed enhanced physicochemical properties compared to the unmodified agarose–chitosan cryomatrix. Deposition of melanin-like moieties on the cryomatrix showed high sorption affinity to uranium. Moreover, the highly porous internal architecture, with interconnected pores and large surface volumes, provided high liquid flow through properties to the cryomatrix, which resulted in providing fast kinetics of uranium adsorption. The cyclic sorption–desorption study suggests reusability of the MAC cryomatrix is possible. This system also facilitates easy separation of floating cryomatrices from aquatic subsurfaces. These results support the potential application of the MAC cryomatrix for the recovery of uranium from contaminated aquatic subsurfaces.Acknowledgements
Author would like to acknowledge Department of Atomic Energy, Government of India, for the financial assistance.References
- Report on Historic Deep Sea Disposal of Radioactive Waste in Layperson's Language. Meeting of the Radioactive Substances Committee (RSC), OSPAR Commission, 2014, RSC14/9/1-E.
- H. Sodaye, S. Nisan, C. Poletiko, S. Prabhakar and P. K. Tewari, Desalination, 2009, 235, 9–32 CrossRef CAS.
- G. Rich and K. Cherry, Hazardous Waste Treatment Technologies, Pudvan Publishers, New York, 1987 Search PubMed.
- A. S. Saini and J. S. Melo, J. Environ. Radioact., 2015, 142, 29–35 CrossRef CAS PubMed.
- K. C. Bhainsa and S. F. D'Souza, J. Environ. Sci. Health, Part A: Environ. Sci. Eng., 2001, 36, 1621–1631 CrossRef CAS.
- A. Mishra, J. S. Melo, D. Sen and S. F. D'Souza, J. Colloid Interface Sci., 2014, 414, 33–40 CrossRef CAS PubMed.
- P. Sar, S. K. Kazy and S. F. D'Souza, Int. Biodeterior. Biodegrad., 2004, 54, 193–202 CrossRef CAS.
- S. V. Bhat, J. S. Melo, B. B. Chaugule and S. F. D'Souza, J. Hazard. Mater., 2008, 158, 628–635 CrossRef CAS PubMed.
- A. Tripathi, J. S. Melo and S. F. D'Souza, J. Hazard. Mater., 2013, 246–247, 87–95 CrossRef CAS PubMed.
- A. S. Saini and J. S. Melo, Bioresour. Technol., 2013, 149, 155–162 CrossRef CAS PubMed.
- A. Tripathi and J. S. Melo, RSC Adv., 2015, 5, 30701–30710 RSC.
- D. Singh, A. Tripathi, S. M. Zo, D. Singh and S. S. Han, Colloids Surf., B, 2014, 116, 502–509 CrossRef CAS PubMed.
- A. Tripathi, A. B. Hadapad, R. S. Hire, J. S. Melo and S. F. D'Souza, Enzyme Microb. Technol., 2013, 53(6–7), 398–405 CrossRef CAS PubMed.
- A. S. Saini, A. Tripathi and J. S. Melo, RSC Adv., 2015, 5, 87206–87215 RSC.
- A. S. Saini, J. Kumar and J. S. Melo, Anal. Chim. Acta, 2014, 849, 50–56 CrossRef CAS PubMed.
- S. B. Savvin, Talanta, 1961, 8, 673–685 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1368 CrossRef CAS.
- H. Freundlich, J. Phys. Chem., 1906, 40, 1361–1368 Search PubMed.
- S. Chegrouche, A. Mellah and S. Telmoune, Water Res., 1997, 31, 1733–1737 CrossRef CAS.
- N. Saleem and H. N. Bhatti, BioResources, 2011, 6(2), 2522–2538 CAS.
- S. R. Distantina, M. Fahrurrozi and W. Wiratni, Eng. J., 2013, 17(3), 57–66 CrossRef.
- C. T. Lee, P. H. Kung and Y. D. Lee, Carbohydr. Polym., 2005, 61, 348–354 CrossRef CAS.
- J. M. Pawelek and S. J. Orlow, Soluble melanin, US 5225435A, 1993, http://www.google.co.in/patents/US5225435.
- M. Tamada, Current Status of Technology for Collection of Uranium from Seawater, Japan Atomic Energy Agency, 2009 Search PubMed.
- N. Seko, A. Katakai, S. Hasegawa, M. Tamada, N. Kasai, H. Takada, T. Sugo and K. Saito, Nucl. Technol., 2003, 144, 274–278 CAS.
- R. A. A. Muzzarelli, Carbohydr. Polym., 2011, 84, 54–63 CrossRef CAS.
- Z. Y. He, B. W. Christopher, Y. T. Zhou, H. L. Nie and L. M. Zhu, Sep. Sci. Technol., 2010, 45, 525–534 CrossRef CAS.
- J. D. Nosanchuk and A. Casadevall, Cell. Microbiol., 2003, 5, 203–223 CrossRef CAS PubMed.
- C. G. Burkhart and C. N. Burkhart, Int. J. Dermatol., 2005, 44, 340–342 CrossRef PubMed.
- J. Mackintosh, J. Theor. Biol., 2001, 211, 101–113 CrossRef CAS PubMed.
- C. J. Luk, J. Yip, C. M. Yuen, C. Kan and K. Lam, J. Fiber Bioeng Informat., 2014, 7, 35–52 Search PubMed.
- R. H. Crist, K. Oberholser, D. Schwartz, J. Marzoff, D. Ryder and D. R. Crist, Environ. Sci. Technol., 1988, 22(7), 755–760 CrossRef CAS PubMed.
- K. Akhtar, A. M. Khalid, M. W. Akhtar and M. A. Ghauri, Bioresour. Technol., 2009, 100, 4551–4558 CrossRef CAS PubMed.
- C. Gok and S. Aytas, J. Hazard. Mater., 2009, 168, 369–375 CrossRef CAS PubMed.
- Y. Yamazaki, Y. Tachibana, T. Kaneshiki, M. Nomura and T. Suzuki, Prog. Nucl. Energy, 2015, 82, 74–79 CrossRef CAS.
- K. Oshita, M. Oshima, Y. Gao, K.-H. Lee and S. Motomizu, Anal. Chim. Acta, 2003, 480, 239–249 CrossRef CAS.
- A. Krestou and D. Panias, Eur. J. Miner. Process. Environ. Prot., 2004, 4, 113–129 Search PubMed.
- M. Gavrilescu, L. V. Pavel and I. Cretescu, J. Hazard. Mater., 2009, 163, 475–510 CrossRef CAS PubMed.
- M. Kalin, W. N. Wheeler and G. Meinrath, J. Environ. Radioact., 2005, 78, 151–177 CrossRef CAS PubMed.
- S. M. Hasany, M. M. saeed and M. Ahmed, J. Radioanal. Nucl. Chem., 2002, 252, 477–484 CrossRef CAS.
- Y. Liu, Y. Liu, X. Cao, R. Hua, Y. Wang, C. Pang, M. Hua and X. Li, J. Radioanal. Nucl. Chem., 2011, 290, 231–239 CrossRef CAS.
- C. J. Luk, J. Yip, C. M. Yuen, C. Kan and K. Lam, J. Fiber Bioeng Informat., 2014, 7(1), 35–52 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04686j |
| This journal is © The Royal Society of Chemistry 2016 |