Synthesis and electrophosphorescence of novel heteroleptic iridium complexes based on thiazole-containing ligands†
Di Liu*,
Ruijuan Yao,
Min Fu,
Deli Li and
Shufen Zhang
State Key Laboratory of Fine Chemicals, School of Chemistry, Dalian University of Technology, 2 Linggong Road, Dalian 116024, China. E-mail: liudi@dlut.edu.cn
First published on 30th March 2016
Abstract
Three novel heteroleptic cyclometalated iridium complexes, namely Ir-Cz, Ir-DBF and Ir-Np, were designed and synthesized for use as emitters in organic light-emitting diodes (OLEDs). 2-Phenyl-(aromatic-fused-thiazole) (the aromatic is carbazole, dibenzofuran or naphthalene) was designed as the major cyclometalating ligand framework to enlarge the ligand conjugate length and to study its influence on the photophysical and electrochemical properties and electroluminescent performances of iridium complexes. The three heteroleptic complexes containing acetylacetonate (acac) as an ancillary ligand emit orange to orange-red phosphorescence with a small bathochromic shift in comparison with the reference complex bis(2-phenylbenzothiozolato-N,C2′)iridium(acetylacetonate) [Ir-Bt]. They all exhibited good performance in phosphorescent OLEDs. In particular, a maximum current efficiency of 54 cd A−1, a peak power efficiency of 34 lm W−1 and an external quantum efficiency of 22.2% were realized for Ir-Np based devices.
Introduction
Transition metal complex based phosphorescent materials are under intensive investigation for their excellent performance when used as emitters in organic light-emitting diodes (OLEDs).1 The theoretical internal quantum efficiency of phosphorescent OLEDs can reach 100% due to their ability to harvest both singlet and triplet excited states.2 Among all the reported phosphors, cyclometalated iridium(III) complexes have been proved to be the most potential phosphorescent materials owing to their high photoluminescence efficiency, relatively short lifetimes and flexible color tunability.3 Various colored iridium complexes have been developed to realize commercial application of OLEDs in full-color flat-panel displays and low-cost solid state lighting. In order to obtain phosphorescent emitters with excellent performances, most of the research works are focused on either exploring novel cyclometalating ligand frameworks or decorating the existing cyclometalating ligands with certain substituents.4Bis(2-phenylbenzothiozolato-N,C2′)iridium(acetylacetonate) [Ir-Bt] and its derivatives are typical yellow to orange phosphors which can be used in combination with blue emitters to fabricate two-emitting-component white OLEDs.5 Due to their high photoluminescence efficiency and excellent performance when used as emitters in OLEDs, researchers have taken great efforts decorating the simple frameworks of the ligands to obtain complexes with better photophysical properties and electrophosphorescence performances.3,6–8 However, most of the modification of Ir-Bt has focused on either replacing the free phenyl ring with other blocks or introducing substitutes on both free phenyl ring and the benzothiazole part. To the best of our knowledge, replacing the phenyl ring of benzothiazole part with other aromatic structure, i.e. building novel aromatic-fused-thiazole structure, has not been reported so far. It is generally believed that increasing the effective conjugation length of a fluorescent molecule would cause a bathochromic shift of the emitting wavelength. However, the impact imposed by changing the ligand conjugate length on phosphorescent emitters is not fully understood, especially for iridium complexes.
In this report, enlightened by the agreeable properties and performance of Ir-Bt and related derivatives, we designed and prepared a group of novel heteroleptic iridium complexes, namely Ir-Cz, Ir-DBF, Ir-Np, with 2-phenyl-(aromatic-fused-thiazole) as major cyclometalating ligand frameworks and acac as ancillary ligand. The new C^N cyclometalating ligands have aromatic ring fused thiazole as the N-coordinating part, in which aromatic ring includes carbazole (Ir-Cz), dibenzofuran (Ir-DBF) and naphthalene (Ir-Np), and phenyl attached at the 2-site of thiazole ring as the C-coordinating part (Scheme 1). The three iridium complexes based on these novel cyclometalating ligands are orange or orange-red emissive, with a red shift of the phosphorescence spectra relative to their reference complex Ir-Bt due to expansion of conjugation length in ligands. The phosphorescent OLEDs made with these complexes emitted efficient orange and orange-red electroluminescence. In particular, a maximum current efficiency of 54 cd A−1 and an external quantum efficiency of 22% were realized for Ir-Np based device, indicating the potential application of these iridium complexes in high performance OLEDs lighting and display.
 | ||
| Scheme 1 Chemical structures and synthetic routes for ligands L-Cz, L-DBF, L-Np and iridium complexes Ir-Cz, Ir-DBF, Ir-Np. | ||
Results and discussion
Synthesis
The chemical structures and synthetic routes of the ligands L-Cz, L-DBF, and L-Np and the target complexes Ir-Cz, Ir-DBF, Ir-Np are shown in Scheme 1. The starting compounds, i.e. the amide 1,9 3,10 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 that were obtained according to the literature methods, were first treated with Lawesson's reagent to generate the intermediates 2, 4, and 6, the following cyclization of which in presence of potassium ferricyanide produced the ligands L-Cz, L-DBF, and L-Np in good yields of 72–81%. The iridium complexes were synthesized through the traditional two-step procedure.12 First, the cyclometalation of the iridium trichloride hydrate with each cyclometalating ligand generated the corresponding dichloro-bridged dimer [Ir(C^N)2Cl]2. Then the target heteroleptic iridium complexes were synthesized via the reaction of the corresponding dichloro-bridged dimer with acetyl acetone in 2-ethoxyethanol in the presence of Na2CO3. The chemical structures of these complexes were characterized and confirmed by 1H NMR and MALDI-TOF mass spectrometry. They have good solubility in common organic solvents so that they could be purified by column chromatography and repeated recrystallization. Finally they were deeply purified by train sublimation to reach a high purity for OLEDs study.
11 that were obtained according to the literature methods, were first treated with Lawesson's reagent to generate the intermediates 2, 4, and 6, the following cyclization of which in presence of potassium ferricyanide produced the ligands L-Cz, L-DBF, and L-Np in good yields of 72–81%. The iridium complexes were synthesized through the traditional two-step procedure.12 First, the cyclometalation of the iridium trichloride hydrate with each cyclometalating ligand generated the corresponding dichloro-bridged dimer [Ir(C^N)2Cl]2. Then the target heteroleptic iridium complexes were synthesized via the reaction of the corresponding dichloro-bridged dimer with acetyl acetone in 2-ethoxyethanol in the presence of Na2CO3. The chemical structures of these complexes were characterized and confirmed by 1H NMR and MALDI-TOF mass spectrometry. They have good solubility in common organic solvents so that they could be purified by column chromatography and repeated recrystallization. Finally they were deeply purified by train sublimation to reach a high purity for OLEDs study.
Photophysical properties
The UV-vis absorption and photoluminescence (PL) spectra of complexes Ir-Cz, Ir-DBF and Ir-Np were measured in dilute CH2Cl2 solution and are shown in Fig. 1. The pertinent data are summarized in Table 1. The absorption of ligands was measured under same conditions for comparison and the spectra are shown in Fig. S1.† As depicted in Fig. 1a, these three complexes show two major absorption bands. The intense absorption bands in the higher energy region (below 370 nm), which appear in the ligands absorption spectra as well (Fig. S1†), are mainly attributed to the spin-allowed ligand centered 1π–π* transitions. The weak absorption bands covering from 370 nm to 550 nm, which appear exclusively in absorption of iridium complexes, can be assigned to the singlet metal-to-ligand charge-transfer (1MLCT) transition and the triplet metal-to-ligand charge-transfer transition (3MLCT) and the spin–orbit coupling enhanced 3π–π* states.13 Upon photoexcitation, complexes Ir-Cz, Ir-DBF and Ir-Np emit intense orange to orange-red phosphorescence with the emission peak at 563–575 nm and a shoulder at 606–616 nm (Fig. 1b). The fine vibronic structures of the emission spectra at room temperature indicate the lowest-energy excited states that are responsible for the light emission may be the mixed triplet excited states of 3MLCT and ligand-centered state 3LC.13 In comparison with the reference compound Ir-Bt, the emission wavelengths of these new complexes all show a certain degree of red shift (Fig. 1b). This may be attributed to the enlargement of the ligands' conjugation length that leads to the energy gap deduction of these complexes. The phosphorescent quantum yields (Φp) were measured in degassed dichloromethane solutions at room temperature and are calculated as 0.51 (Ir-Cz), 0.35 (Ir-DBF), and 0.53 (Ir-Np). | ||
| Fig. 1 (a) UV-vis absorption and (b) PL spectra of complexes Ir-Cz, Ir-DBF and Ir-Np in dilute CH2Cl2 solutions at 293 K. | ||
| Compound | λabsa (nm) | λema (nm) | Φpb | Eoxonsetc [V] | LUMO [eV] | HOMOc [eV] | Egd [eV] |
|---|---|---|---|---|---|---|---|
| a Measured in dichloromethane (1.0 × 10−5 mol L−1) at room temperature.b Φp: phosphorescent quantum yields measured in degassed dichloromethane solutions using a Hamamatsu absolute PL quantum yield spectrometer C11347.c Eoxonset: the onset potential of first oxidation wave was taken in dichloromethane solution, and HOMO level was determined using the Eoxonset value.d Optical band gap determined by the absorption edge of fresh film sample.e The parameters of reference compound It-Bt were measured under identical conditions to the present complexes. | |||||||
| Ir-Cz | 303, 370, 400, 445 | 575 (616 sh) | 0.51 | 0.73 | −3.05 | −5.13 | 2.08 |
| Ir-DBF | 299, 322, 403, 446 | 561 (598 sh) | 0.35 | 0.83 | −3.09 | −5.23 | 2.14 |
| Ir-Np | 276, 336, 363, 405, 457 | 568 (607 sh) | 0.53 | 0.85 | −3.15 | −5.25 | 2.10 |
| Ir-Bte | 327, 354, 406, 443, 474 | 556 (590 sh) | 0.49 | 0.87 | −3.11 | −5.27 | 2.16 |
Electrochemical properties
The electrochemical behaviors of these iridium complexes were investigated by cyclic voltammetry (CV) measurements using a conventional three-electrode cell set-up with 0.1 M tetra(n-butyl)ammonium hexafluorophosphate (Bu4NPF6) as a supporting electrolyte in CH2Cl2. The cyclic voltammograms are shown in Fig. 2. During anodic scanning, all these complexes show reversible oxidation waves. Ir-Cz underwent three reversible oxidation processes, with the peak oxidation potentials at 0.86, 1.09, and 1.46 V vs. a saturated calomel electrode (SCE), respectively. And Ir-DBF exhibited two reversible oxidation waves with peak potentials at 0.95 and 1.69 V. While Ir-Np shown only one reversible oxidation process with peak potential at 0.93 V. These oxidation processes can be assigned to the metal-centered IrIII/IrIV oxidation couple.14 In addition, based on the obvious electron-donating feature of carbazole and dibenzofuran groups, the additional oxidation processes for these iridium complexes are also possible due to the oxidation of these units on ligands. The onset potential (Eoxonset) of the first oxidation wave was used to determine the highest occupied molecular orbital (HOMO) level according to the empirical equation: EHOMO = −e(Eoxonset + 4.4).4,15 And the lowest unoccupied molecular orbital (LUMO) energy levels were estimated from the HOMO energy levels and the optical energy band gaps (Eg) that was obtained by absorption edge of the complex films (Fig. S1d†).4 All the relevant data are listed in Table 1. The HOMO levels of these complexes are in the range of −5.13 eV to −5.25 eV, and the LUMO are in −3.05 eV to −3.15 eV. The HOMO energy levels of these three complexes slightly increase comparing with the reference iridium complex Ir-Bt that was measured under identical conditions as well (EHOMO = −5.27 eV, ELUMO = −3.11 eV).15 Apparently the attachment of electron-rich groups, such as carbazole, dibenzofuran and naphthene, to the ligands not only enlarged the conjugation length of the ligands and complexes but also improves the electron density of the complexes that finally leads to elevation of EHOMO and ELUMO at the same time.Phosphorescent organic light-emitting diodes
The electroluminescence properties of complexes Ir-Cz, Ir-DBF and Ir-Np were investigated by using them as doped emitters in OLEDs. The devices have the configuration of ITO/PEDOT:PSS (45 nm)/TCTA (10 nm)/Ir:CBP (8 wt%, 30 nm)/TPBI (45 nm)/LiF (1 nm)/Al (150 nm). The structures of the OLEDs and all the chemicals used are shown in Fig. S2.† In these devices, PEDOT:PSS (poly(styrene sulfonate):poly(3,4-ethylenedioxythiophene)) acts as hole-injecting and -transporting layer, TCTA (4,4′,4′′-tri(9-carbazoyl)triphenylamine) as a hole-transporting/electron blocking material, CBP (4,4′-N,N′-dicarbazolebiphenyl) as the host material, TPBI (1,3,5-tris[N-(phenyl)benzimidazole]-benzene) as an electron-transporting/hole blocking material, and LiF as the electron-injecting layer. The EL properties of the reference compound Ir-Bt was also studied under identical conditions to evaluate the EL performance of these complexes.The devices based on Ir-Cz, Ir-DBF and Ir-Np exhibited intense orange to orange-red emission at the doping concentration of 8 wt% with the Commission International de L'Eclairage (CIE) color coordinates of (0.55, 0.44), (0.50, 0.49), and (0.54, 0.46), respectively. As shown in Fig. 3a, the EL spectra are almost consistent with their PL (Fig. 1b) in terms of spectral profile and wavelength position, indicating that the EL emission originates from the triplet excited state of these complexes. Meanwhile, the absence of any residual emission from TCTA, CBP or TPBI suggests a complete energy transfer from the host to dopant molecules and efficient confinement of the triplet excitons on dopant molecules. Additionally, as shown in Fig. S3† (taking Ir-DBF device as an example), the EL spectra were almost independent of the applied voltages, indicating stable emission properties of these devices. The luminance–voltage–current density (L–V–J) curves of these devices are shown in Fig. 3b. These three complexes based devices turned on (to deliver a brightness of 1 cd m−2) at 3.7 to 4.1 V, and exhibited the maximum brightness of 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 to 48
320 to 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 cd m−2 at 10 or 11 V. It is clear that the Ir-Np based device exhibited remarkably higher current density and brightness under a given voltage than all other devices, which is probably because Ir-Np is more favorable for charge transportation and higher current density than other analogues especially when the doping concentration is as high as 8 wt%.
820 cd m−2 at 10 or 11 V. It is clear that the Ir-Np based device exhibited remarkably higher current density and brightness under a given voltage than all other devices, which is probably because Ir-Np is more favorable for charge transportation and higher current density than other analogues especially when the doping concentration is as high as 8 wt%.
Fig. 4 and S4† illustrate the efficiency curves for these devices. The Ir-Cz based device exhibited a peak current efficiency (or luminance efficiency, ηL) of 35.3 cd A−1, corresponding to a peak power efficiency (ηP) of 18.5 lm W−1 and a maximum external quantum efficiency (ηext) of 16.4%. In comparison, the Ir-DBF and Ir-Np based devices exhibited better performance. For example, a maximum ηL of 46 cd A−1 (corresponding to a ηP of 24 lm W−1 and a ηext of 16.9%) was obtained for the Ir-DBF device. In particular, the Ir-Np device gave the best performance with the maximum efficiencies of 54 cd A−1, 34 lm W−1 and 22.2%. Under identical conditions, the reference complex Ir-Bt based device realized the maximum efficiencies of 39 cd A−1, 24 lm W−1 and 12.9%. All the EL data are summarized in Table 2. Apparently the novel complexes Ir-DBF and Ir-Np achieved much better performance than their reference Ir-Bt, implying that enlarging the conjugating structures of the cyclometalating ligands may be an effective strategy to both make bathochromic shift of the emission and improve light-emitting performance for the cyclometalated iridium complexes.
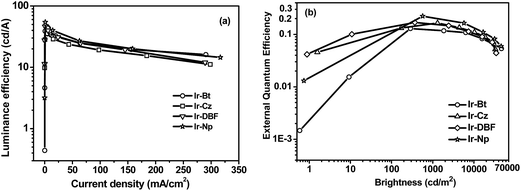 | ||
| Fig. 4 The EL efficiencies curves for the iridium complexes based OLEDs. (a) Luminance efficiency versus current density. (b) External quantum efficiency versus brightness. | ||
| Phosphor | Von (V) | Jmax (mA cm−2, V) | Lmax (cd m−2, V) | ηL (cd A−1) | ηP (lm W−1) | ηext [%] | λEL (nm) | CIE (x, y) |
|---|---|---|---|---|---|---|---|---|
| Ir-Cz | 3.7 | 298, 11 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320, 11 320, 11 |
35.3 | 18.5 | 16.4 | 575 (622 sh) | (0.55, 0.44) |
| Ir-DBF | 4.1 | 289, 11 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780, 11 780, 11 |
46 | 24 | 16.9 | 563 (605 sh) | (0.50, 0.49) |
| Ir-Np | 4.1 | 531, 11 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, 10 200, 10 |
54 | 34 | 22.2 | 573 (617 sh) | (0.54, 0.46) |
| Ir-Bt | 3.6 | 290, 10 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860, 11 860, 11 |
39 | 24 | 12.9 | 560 (600 sh) | (0.48, 0.54) |
Conclusion
In conclusion, three novel orange-emitting heteroleptic iridium complexes, Ir-Cz, Ir-DBF and Ir-Np, containing thiazole moiety in cyclometalating ligands have been designed and synthesized for application in OLEDs. In comparison with the reference complex Ir-Bt based on 2-phenyl-benzothiazole ligands, replacement of the benzene ring with the electron-rich carbazole, dibenzofuran, and naphthene rings in ligands can not only enlarge the π-conjugation and consequently red shift the phosphorescence, but also cause the elevation of the HOMO and LUMO levels of the iridium complexes. However, it is obvious that the bathochromic shift caused by the structure modification in this way is only limited probably because the HOMO/LUMO of the iridium complex molecules have small distribution on these positions. The phosphorescent OLEDs with these iridium complexes as doped emitters all exhibited orange or orange-red electroluminescence with good performance. In particular, the Ir-Np based orange-red OLED realized a high luminance efficiency of 54 cd A−1, a maximum power efficiency of 34 lm W−1 and an external quantum efficiency of 22.2%, which are better than the performance of the reference complex Ir-Bt. These novel iridium complexes may find potential application as orange to orange-red emitters to fabricate both single-color and blue-orange two-emitting-component white OLEDs.Experimental
Measurement and characterization
1H NMR spectra were recorded on a 400 MHz Varian Unity Inova spectrophotometer. Mass spectra were taken on MALDI micro MX and HP1100LC/MSD MS spectrometers. The photoluminescence and UV-vis absorption spectra measurements were performed on a Perkin-Elmer LS55 spectrometer and a Perkin-Elmer Lambda 35 spectrophotometer, respectively. The phosphorescence quantum yields of these iridium complexes in dilute oxygen-free dichloromethane solutions were measured with a Hamamatsu absolute PL quantum yield spectrometer C11347. The electrochemical measurements of these iridium complexes were carried out by using a conventional three-electrode configuration (a glass carbon working electrode, a Pt-wire counter electrode, and a saturated calomel reference electrode (SCE)) on an electrochemical workstation (BAS100B, USA) at a scan rate of 100 mV s−1. All measurements were made on deoxygenated dichloromethane solutions with 0.1 M [Bu4N]PF6 as the electrolyte at room temperature.OLEDs fabrication and measurements
The pre-cleaned ITO glass substrates with a sheet resistance of 15 Ω per square were treated by UV-ozone for 20 min. A 45 nm thick PEDOT:PSS film was first deposited on ITO glass substrates, and baked at 120 °C for 30 min in air. Then, the substrate was transferred into a vacuum chamber for deposition of organic layers at a base pressure less than 10−6 torr. First, a 10 nm thick TCTA was deposited on PEDOT:PSS film. For all the OLEDs, the emitting layers were then deposited by co-evaporation of the iridium complex and the CBP host. Successively, TPBI, LiF and Al were evaporated and deposited. The EL spectra, CIE coordinates and L–V–J curves of the devices were measured with a PR705 photometer and a source-measure-unit Keithley236 under ambient conditions. The forward viewing external quantum efficiency (ηext) was calculated using the current efficiency, EL spectra and human photopic sensitivity.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5) as eluent to yield pure intermediate 2, 4, or 6 as yellow granular solid, which was directly put into next step reaction.
3.5) as eluent to yield pure intermediate 2, 4, or 6 as yellow granular solid, which was directly put into next step reaction.To a warm (50 °C) aqueous solution of potassium ferricyanide (20 wt%, 4 mmol) was added a solution of intermediate 2, 4, or 6 (1 mmol) in 10 wt% sodium hydroxide (8 mmol) and several drops of ethanol in batches. The reaction mixture was heated to 90 °C and stirred for 2 h, then cooled to room temperature and water (100 mL) was added. Extraction with dichloromethane (3 × 50 mL) followed by evaporation of solvent produced the crude product residue, which was then separated by column chromatography over silica gel with petroleum ether/dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent to give pure ligand products.
4) as the eluent to give pure ligand products.
L-Cz. Yield 72%. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.18–8.13 (m, 4H), 7.55–7.47 (m, 6H), 7.38 (t, 1H), 4.46–4.40 (m, 2H), 1.48 (t, 3H). TOF-EI-MS (m/z): 328.1033 ([C21H16N2S]+).
L-DBF. Yield 84%. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.19–8.15 (m, 3H), 8.02 (d, J = 8.2 Hz, 1H), 7.75 (d, J = 8.8 Hz, 1H), 7.68 (d, J = 8.2 Hz, 1H), 7.56–7.51 (m, 4H), 7.49 (d, J = 7.4 Hz, 1H). TOF-EI-MS (m/z): 301.0558 ([C19H11NOS]+).
L-Np. Yield 81%. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.16–8.14 (m, 2H), 8.12 (d, J = 8.8 Hz, 1H), 8.05 (d, J = 8.0 Hz, 1H), 7.98 (d, J = 8.2 Hz, 1H), 7.89 (d, J = 9.0 Hz, 1H), 7.62–7.59 (t, 1H), 7.57 (d, J = 6.8 Hz, 1H), 7.53–7.49 (m, 3H). TOF-EI-MS (m/z): 261.0620 ([C17H11NS]+).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as eluent to yield the pure product of the desired iridium complex.
4) as eluent to yield the pure product of the desired iridium complex.
Ir-Cz. Yield 35%. MALDI-TOF-MS (m/z): 946.2756 ([C47H37IrN4O2S2]+). 1H NMR (400 MHz, CDCl3) δ (ppm) 8.31 (d, J = 9.0 Hz, 2H), 8.24 (d, J = 7.6 Hz, 2H), 7.75 (d, J = 8.0 Hz, 2H), 7.60–7.57 (m, 4H), 7.52 (d, J = 9.0 Hz, 2H), 7.45–7.42 (t, 2H), 6.90–6.86 (t, 2H), 6.61–6.57 (t, 2H), 6.47 (d, J = 8.0 Hz, 2H), 5.17 (s, 1H), 4.53–4.47 (m, 4H), 1.82 (s, 6H), 1.54–1.50 (t, 6H). Anal. calcd for C47H37IrN4O2S2: C, 59.66; H, 3.94; N, 5.92; S, 6.78. Found: C, 59.61; H, 3.96; N, 5.89; S, 6.74.
Ir-DBF. Yield 45%. MALDI-TOF-MS (m/z): 892.0980 ([C43H27IrN4O2S2]+). 1H NMR (400 MHz, CDCl3) δ (ppm) 8.29 (d, J = 9.0 Hz, 2H), 8.11 (d, J = 6.8 Hz, 2H), 7.77 (d, J = 7.0 Hz, 2H), 7.72–7.66 (m, 4H), 7.60 (d, J = 8.2 Hz, 2H), 7.56–7.52 (t, 2H), 6.93–6.89 (t, 2H), 6.66–6.62 (t, 2H), 6.48 (d, J = 8.0 Hz, 2H), 5.16 (s, 1H), 1.80 (s, 6H). Anal. calcd for C43H27IrN4O2S2: C, 58.16; H, 3.06; N, 6.31; S, 7.22. Found: C, 58.12; H, 3.09; N, 6.28; S, 7.20.
Ir-Np. Yield 70%. MALDI-TOF-MS (m/z): 812.1436 ([C39H27IrN2O2S2]+). 1H NMR (400 MHz, CDCl3) δ (ppm) 8.20 (d, J = 9.0 Hz, 2H), 8.12 (d, J = 7.8 Hz, 2H), 7.99 (d, J = 8.2 Hz, 2H), 7.85 (d, J = 9.0 Hz, 2H), 7.74 (d, J = 9.0 Hz, 2H), 7.70–7.66 (t, 2H), 7.61–7.57 (t, 2H), 6.89–6.85 (t, 2H), 6.48 (d, J = 7.8 Hz, 2H), 5.13 (s, 1H), 1.78 (s, 6H). Anal. calcd for C39H27IrN2O2S2: C, 57.69; H, 3.35; N, 3.45; S, 7.90. Found: C, 57.63; H, 3.33; N, 3.42; S, 7.88.
Acknowledgements
We thank the National Natural Science Foundation of China (21274016, 21374013, and 21421005), the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13R06) and Program for DUT Innovative Research Team (DUT2013TB07), and the Fundamental Research Funds for the Central Universities (DUT15YQ101 and DUT13LK06) for financial support of this work.References
- (a) H. Yersin, Top. Curr. Chem., 2004, 241, 1 CrossRef CAS; (b) E. Holder, B. M. W. Langeveld and U. S. Schubert, Adv. Mater., 2005, 17, 1109 CrossRef CAS; (c) P.-T. Chou and Y. Chi, Eur. J. Inorg. Chem., 2006, 17, 3319 CrossRef; (d) P.-T. Chou and Y. Chi, Chem.–Eur. J., 2007, 13, 380 CrossRef CAS PubMed.
- B. Ma, P. I. Djurovich and M. E. Thompson, Coord. Chem. Rev., 2005, 249, 1501 CrossRef CAS.
- (a) R. Wang, D. Liu, H. Ren, T. Zhang, H. Yin, G. Liu and J. Li, Adv. Mater., 2011, 23, 2823 CrossRef CAS PubMed; (b) X. Wang, S. Wang, Z. Ma, J. Ding, L. Wang, X. Jing and F. Wang, Adv. Funct. Mater., 2014, 24, 3413 CrossRef CAS; (c) D. Xia, B. Wang, B. Chen, S. Wang, B. Zhang, J. Ding, L. Wang, X. Jing and F. Wang, Angew. Chem., Int. Ed., 2014, 53, 1048 CrossRef CAS PubMed; (d) Y. Wang, S. Wang, N. Zhao, B. Gao, S. Shao, J. Ding, L. Wang, X. Jing and F. Wang, Polym. Chem., 2015, 6, 1180 RSC.
- R. Wang, D. Liu, R. Zhang, L. Deng and J. Li, J. Mater. Chem., 2012, 22, 1411 RSC.
- S. Lamansky, P. Djurovich, D. Murphy, F. Abdel-Razzaq, H.-E. Lee, C. Adachi, P. E. Burrows, S. R. Forrest and M. E. Thompson, J. Am. Chem. Soc., 2001, 123, 4304 CrossRef CAS PubMed.
- W. Chang, A. T. Hu and J. Duan, J. Organomet. Chem., 2004, 689, 4882 CrossRef CAS.
- I. R. Laskar and T.-M. Chen, Chem. Mater., 2004, 16, 111 CrossRef CAS.
- X. Wei, J. Peng and J. Cheng, Adv. Funct. Mater., 2007, 17, 3319 CrossRef CAS.
- J. B. Kyziol and W. P. Wozniak, Pol. J. Chem., 1981, 55, 937 CAS.
- R. G. Johnson, H. B. Willish and H. K. Gilman, J. Am. Chem. Soc., 1954, 76, 6407 CrossRef CAS.
- D. D. Young, C. M. Connelly, C. Grohmann and A. Deiters, J. Am. Chem. Soc., 2010, 132, 7976 CrossRef CAS PubMed.
- S. Lamansky, P. Djurovich, D. Murphy, F. Abdel-Razzaq, R. Kwong, I. Tsyba, M. Bortz, B. Mui, R. Bau and M. E. Thompson, Inorg. Chem., 2001, 40, 1704 CrossRef CAS PubMed.
- W.-Y. Wong, G.-J. Zhou, X.-M. Yu, H.-S. Kwok and B.-Z. Tang, Adv. Funct. Mater., 2006, 16, 838 CrossRef CAS.
- M. K. Nazeeruddin, R. T. Wegh, Z. Zhou, C. Klein, Q. Wang, F. DeAngelis, S. Fantacci and M. Gratzel, Inorg. Chem., 2006, 45, 9245 CrossRef CAS PubMed.
- R. Wang, L. Deng, T. Zhang and J. Li, Dalton Trans., 2012, 41, 6833 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures. See DOI: 10.1039/c6ra04551k |
| This journal is © The Royal Society of Chemistry 2016 |


