The degradation of mechanical properties due to stress concentration caused by retained acetone in epoxy nanocomposites
R. Atif,
I. Shyha and
F. Inam*
Department of Mechanical and Construction Engineering, Faculty of Engineering and Environment, Northumbria University, City Campus, Newcastle upon Tyne, NE1 8ST, UK. E-mail: fawad.inam@northumbria.ac.uk
First published on 30th March 2016
Abstract
Multi-layered graphene (MLG)–epoxy nanocomposites of three different types were produced using the solution casting technique with MLG dispersed in three different mediums; acetone (MA), an epoxy (ME), and a hardener (MH). In the case of MLG dispersed in the hardener (MH), the maximum increases in tensile and flexural properties, fracture toughness, and microhardness were observed at 0.3 wt% of MLG. The Young's modulus increased from 610 MPa to 758 MPa (24% increase) and the tensile strength increased from 46 MPa to 60 MPa (31% increase). The fracture toughness (K1C) increased from 0.8 MPa m1/2 to 1.1 MPa m1/2 (29% increase) and the Charpy impact toughness increased from 0.85 kJ m−2 to 1.61 kJ m−2 (89% increase). An increase in the storage modulus and glass transition temperature (Tg) was also observed which is attributed to the high stiffness and restriction of polymer chains. Also, if the acetone is not completely removed, the products would have porosity which acts as a stress concentrator and significantly degrades the mechanical properties of the nanocomposites.
Introduction
There has been an exponential rise in the use of graphene in various research disciplines mainly because of the properties inherited from, and transferred by, graphene to the processed graphene based materials.1,2 After the ground-breaking experiments on the two dimensional material graphene by Nobel Laureates, Sir Andre Geim and Konstantin Novoselov3 from the University of Manchester, graphene came into the limelight in the research field mainly because of its excellent electrical,4 thermal,5 and mechanical properties.6 Graphene has found widespread applications in electronics,7 bio-electric sensors,8 energy technology,9 lithium batteries,10 aerospace,11 bio-engineering,12 and other fields of nanotechnology.13Graphene dispersion is one of the key factors defining the performance of graphene based polymer nanocomposites.1,14 Uniformly dispersed graphene shares external loads and blocks the advancing cracks, which elevate the mechanical properties. Poorly dispersed graphene acts as stress raiser and causes stress concentration, which deteriorate the mechanical properties. In pristine form, graphene tends to agglomerate due to weak van der Waals interactions.15 To avoid aggregation and achieve uniform dispersion, various methods are employed. Some of them include dispersion methods, functionalization, volume fraction, and dispersion medium. Among various dispersion methods, mechanical stirring,16 sonication,17 and calendering processes18 are most commonly used. In functionalization, functional groups are attached to the surface of graphene using two main methods; (1) physical or non-covalent functionalization, and (2) chemical or covalent functionalization.19,20 The volume fraction of graphene conspicuously affects the mechanical properties as a very low content of graphene cannot share much of the applied load while a very high content of graphene makes uniform dispersion difficult.21 Another way of improving the dispersion state is the use of a better dispersion medium.17 It was shown that dispersing graphene in a suitable organic solvent prior to dispersion in the resin significantly improves the dispersion state and begets a concomitant rise in the mechanical properties.22
The dispersion solvent was selected for two main characteristics; (1) low viscosity of solvent and (2) ability to lower the viscosity of polymer matrix as dispersion becomes easy in a low viscosity medium. However, lower mechanical properties were reported in some cases when an organic solvent was used as dispersion medium.23–27 Loos et al.28 produced epoxy samples with varied amount of acetone (0, 7, 10, 13 wt%). They reported significant drop in Young's modulus, tensile strength, and fracture strain as a result of residual acetone. The drop in mechanical properties was found directly related with the amount of acetone used.28 The traces of organic solvents influence cure kinetics and restrict the cross-linking process.29 Hong and Wu30 mentioned that residues of organic solvents result in lower curing exotherm, reaction rate, initial curing rate, glass transition temperature (Tg), and reaction order. They also reported that organic solvents with higher boiling points have greater effect on cure kinetics and mechanical properties of epoxy.30
Therefore, the use of solvent is not completely propitious that can be attributed to four main reasons; (1) some organic solvents are not efficient dispersants for graphene, (2) the remnants of organic solvent adversely affect the cure kinetics, (3) any residues of the solvent cause porosity which is detrimental to the mechanical properties, and additionally, (4) the solvent needs to be evaporated after dispersion which delays the process and increases the cost. Previously, the organic solvents were used as dispersion mediums, especially for carbon nanotubes to improve their dispersion state in polymer matrix.31–35 This practice was justified as the cylindrical shape and very high aspect ratio of carbon nanotubes caused them to entangle severely. Also, dispersing them in polymer resin with relatively high viscosity was quite difficult. Therefore, the use of organic solvents was inevitable. The same idea has been applied for multi-layered graphene (MLG) without considering the difference in the shapes and dimensions. As MLG is flat in shape, it can be speculated that the entanglement of MLG would not be as severe as that of carbon nanotubes. However, this hypothesis requires experimental corroboration.
The objective of this work was to study the damage tolerance and fracture toughness of MLG–epoxy nanocomposites with and without the use of organic solvent (acetone in this case) as dispersion medium. The MLG was dispersed using tip sonication in three different mediums; (1) acetone (MA), (2) epoxy (ME), and (3) hardener (MH). As epoxy and hardener had widely different viscosities, it was expected that dispersion state of MLG would be different. The epoxy was dense while the hardener was very fluid. This was one of the reasons that MLG showed better dispersion in the hardener than in the epoxy.6 The findings showed significant impact of dispersion medium on overall properties of the produced nanocomposites. It was observed that retained acetone caused porosity that acted as stress raiser and degraded the mechanical properties of produced nanocomposites.
Experimental section
Materials
Multi-layered graphene (MLG) of 12 nm average thickness and 4.5 μm average lateral size with specific surface area of 80 m2 g−1 and purity 99.2% was purchased from Graphene Supermarket. MLG was washed extensively with acetone to remove any impurities and tip sonicated for 6 h to fragment any aggregates. Bisphenol A-epichlorohydrin based epoxy resin, having a density of ∼1.3 g cm−3, was purchased from Polyfibre, UK. Dimethylbenzylamine isophorone diamine based low viscosity fast curing hardener with ∼1.1 g cm−3 density was used to cure the epoxy and purchased from Polyfibre, UK. The low viscosity of the hardener helped improve the dispersion state and the fast curing helped prevention of agglomeration of MLG. The gelation time of the resin was 43 min at room temperature. Acetone of purity 99.8% was purchased from Sigma-Aldrich and was used as dispersion medium for MLG.Samples production
MLG of different weight fractions (0.1 wt%, 0.3 wt%, 0.5 wt% and 1.0 wt%) were taken and dispersed in three different mediums; (1) acetone (MA), (2) epoxy (ME), and (3) hardener (MH). The MLG was dispersed using tip sonication for 3 h. The sonication was carried out using tip sonicator of power 750 W and frequency 250 kHz (Vibra-cell model VC 750, USA). The operation mode was 70% power with 10 s vibration and 5 s break. Although the sonication was carried out at room temperature, however, temperature of the system rose due to high energy vibration produced by tip sonicator. The acetone was removed at 60 °C for 2 h. The other part of resin was added in epoxy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hardener ratio of 2
hardener ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Following thorough hand mixing for 10 min, vacuum degassing was carried out for 15 min. The resin was poured into silicone molds (without any release agent) and cured at room temperature for 6 h followed by post-curing at 80 °C for 6 h.
1. Following thorough hand mixing for 10 min, vacuum degassing was carried out for 15 min. The resin was poured into silicone molds (without any release agent) and cured at room temperature for 6 h followed by post-curing at 80 °C for 6 h.
Characterization
Samples densities were calculated according to ASTM Standard D792. The densities of epoxy, hardener, MLG, and water were, 1.3, 1.1, 2.26, and 0.9975 g cm−3, respectively. The weight of samples in air and water were measured in Sartorius MC210S analytical balance (with the readability of 0.01 mg). Experimental density and densification were calculated using eqn (1) and (2) respectively.
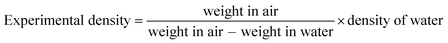 | (1) |
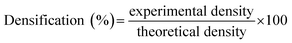 | (2) |
Vickers microhardness tests were conducted using Buehler Micromet II to determine the hardness values of nanocomposites. The load of 200 g was applied for 10 seconds. Tensile, three point bend, and fracture toughness tests were conducted using an Instron Universal Testing Machine (model 3382). The displacement rate was kept 1 mm min−1 for all three tests. Five specimens were tested for each composition.
The schematics of mechanical test specimens are shown in Fig. 1. Tensile properties were measured according to ASTM D638 Type-V geometry with specimen thickness 4 mm. Three point bend test was conducted according to ASTM D790 with specimen dimensions 3 × 12.7 × 48 mm. A single-edge-notch three point bending (SEN-TPB) specimen was used to determine mode-I fracture toughness (K1C) according to ASTM D5045. The specimen dimensions were 3 × 6 × 36 mm with a crack length of a = 3 mm. The notch was made at the mid of sample and tapped to sharpen by a fresh razor blade. The K1C was calculated using eqn (3),
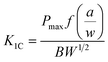 | (3) |
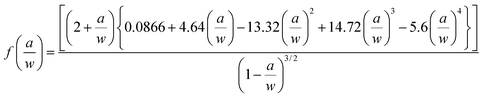 | (4) |
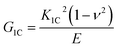 | (5) |
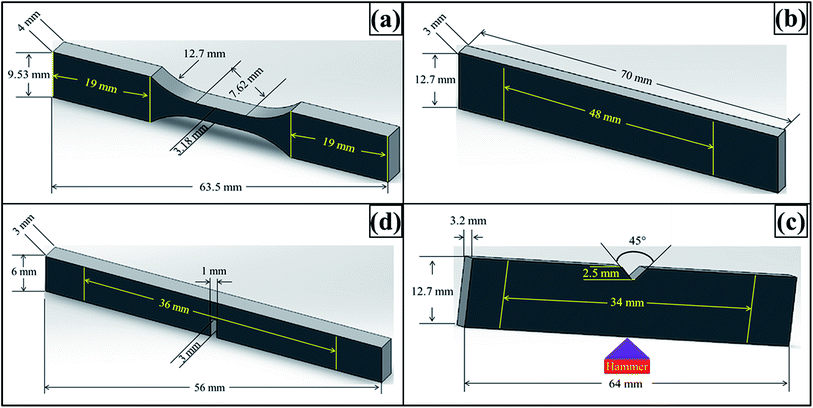 | ||
| Fig. 1 Schematics of specimens: (a) tensile, (b) flexural, (c) Charpy impact toughness, and (d) SEN-TPB. | ||
Charpy impact toughness test was carried out according to ASTM D6110 using notched specimen with dimensions 3.2 × 12.7 × 64 mm. A V-notch (45°) was made in the middle of the specimen whose depth was 2.5 mm and tip radius was 0.25 mm. The specimen was placed as simply supported beam and hit by hammer from behind the notch. The impact toughness was calculated using eqn (6),
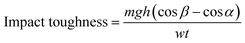 | (6) |
where, m is hammer mass (kg), g is standard gravity (9.8 m s−2), h is length of hammer arm (m), β is hammer swing up angle after test piece breaks (rad), α is hammer lifting up angle (rad), w is sample width (mm), and t is sample thickness (mm).
An Alicona Infinite Focus optical microscope (G4) was used to generate optical micrographs and measure pore size of nanocomposites. The samples were ground and polished using diamond paste of 3 μm size. DMA (model 8000, PerkinElmer) was used to determine dynamic storage modulus (É), and loss modulus (E′′) of the samples. The loss factor tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ was calculated as the ratio (E′′/É). Rectangular test specimens of dimensions 2.5 × 8 × 30 mm were used with a single cantilever clamp. All tests were carried out by the temperature sweep method (temperature ramp from 30 °C to 180 °C at 10 °C min−1) at a constant frequency of 1 Hz. The glass transition temperature (Tg) was taken as the temperature value at the peak of tan
δ was calculated as the ratio (E′′/É). Rectangular test specimens of dimensions 2.5 × 8 × 30 mm were used with a single cantilever clamp. All tests were carried out by the temperature sweep method (temperature ramp from 30 °C to 180 °C at 10 °C min−1) at a constant frequency of 1 Hz. The glass transition temperature (Tg) was taken as the temperature value at the peak of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves. Scanning electron microscopy analysis using a SEM FEI Quanta 200, was carried out on the fractured surfaces of tensile specimens to evaluate the fracture modes in the nanocomposites. The fractured portions were cut from the specimens and a layer of gold was applied using Emscope sputter coater model SC500A.
δ curves. Scanning electron microscopy analysis using a SEM FEI Quanta 200, was carried out on the fractured surfaces of tensile specimens to evaluate the fracture modes in the nanocomposites. The fractured portions were cut from the specimens and a layer of gold was applied using Emscope sputter coater model SC500A.
Results and discussion
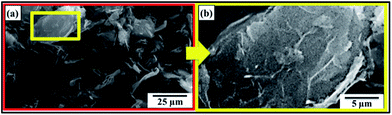
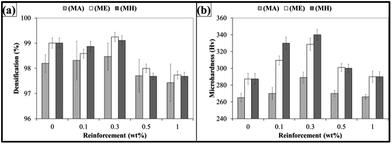
The SEM micrographs of MLG are shown in Fig. 2. The MLG has a large aspect ratio. This wrinkled structure and topographical features can significantly improve the interfacial interactions with polymer chains.20 The densification percentage of MLG–epoxy nanocomposites versus weight fraction of MLG is shown in Fig. 3(a). Large standard deviations in densification values of nanocomposites with acetone indicate non-uniform dispersion of MLG and porosity. In addition, there is some porosity in nanocomposites without acetone that shows that there is some air entrapment and restriction in the movement of polymer chains due to MLG. Another reason for this porosity is the fast curing of epoxy resin as the volatiles could not get enough time to escape during curing.28 The Vickers microhardness values of nanocomposites are shown in Fig. 3(b). The maximum increase was observed with MLG in hardener (MH) up to 18% at 0.3 wt% of MLG. The MLG tends to fill in the porosity and restricts the movement of polymer chains that increase the microhardness. A drop in densification and microhardness after 0.3 wt% indicate that MLG did not fill in pores and may have prevented the escape of volatiles.36The tensile and flexural properties of the produced nanocomposites are shown in Fig. 4(a)–(f). Fig. 4(a) shows variation in Young's modulus where the maximum increase was observed in case of MLG in hardener (MH) up to 24% at 0.3 wt% of MLG. Fig. 4(b) shows variation in Ultimate Tensile Strength (UTS). The UTS increased at 0.1 wt% of MLG in all three cases (MA, ME, and MH) and decreased afterwards. The maximum increase was observed in case of MLG in hardener (MH) up to 32%. Fig. 4(c) shows variation in tensile strain (%) which is percent value of strain at UTS. Fig. 4(d) shows variation in flexural modulus. The flexural modulus increased with increasing weight fraction of reinforcement in all three cases up to 0.3 wt% with maximum increase observed in case of MLG in hardener (MH) up to 46%. Fig. 4(e) shows variation in flexural strength. The flexural strength increased at 0.1 wt% of MLG and decreased afterwards. The maximum increase was observed in case of MLG in hardener (MH) up to 30%. Fig. 4(f) shows variation in flexural strain (%) which is percent value of strain at flexural strength. The flexural strain (%) decreased in all three cases with increasing weight fraction of MLG.
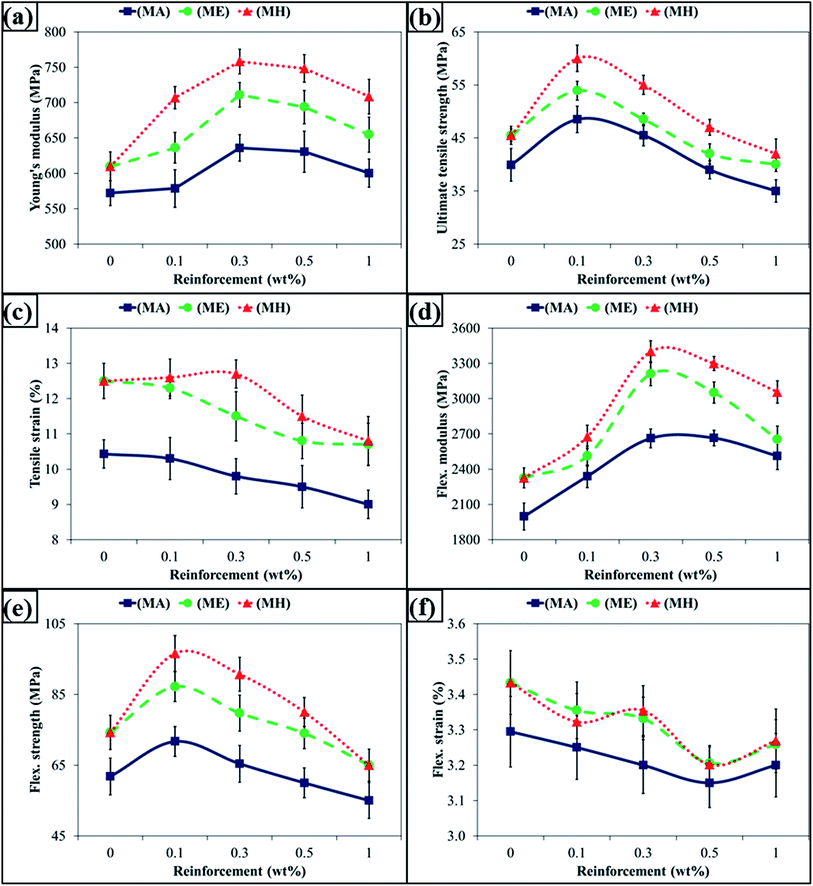 | ||
| Fig. 4 Tensile and flexural properties: (a) Young's modulus, (b) ultimate tensile strength, (c) tensile strain (%), (d) flexural modulus, (e) flexural strength, and (f) flexural strain (%). | ||
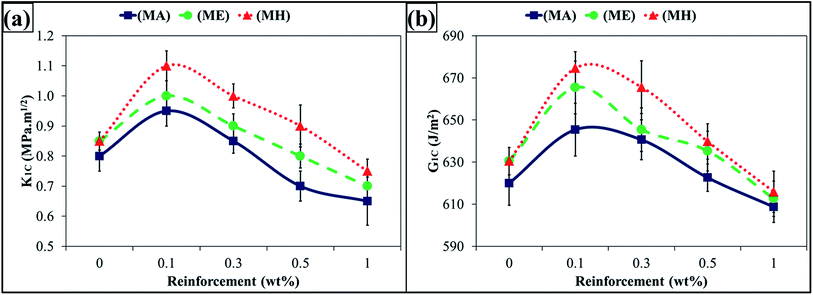
The K1C and G1C values are shown in Fig. 5(a) and (b), respectively. The K1C increased at 0.1 wt% of MLG and decreased afterwards. The maximum increase was observed in case of MLG in hardener (MH) up to 29%. The G1C increased at 0.1 wt% of MLG and decreased afterwards. The maximum increase was observed in case of MLG in hardener (MH) up to 6.9%. An improvement in fracture toughness with MLG was also reported by Rafiee et al. and reported that graphene platelets significantly out-perform carbon nanotube additives.37 A similar results were reported by Wan et al. where K1C increased up to 25.6% at 0.25 wt% of graphene oxide.38 The variation in Charpy impact toughness values is shown in Fig. 6. The Charpy impact toughness significantly increased and maximum improvement was observed at 0.3 wt% of MLG and decreased afterwards. The maximum increase was observed in case of MLG in hardener (MH) up to 89%.
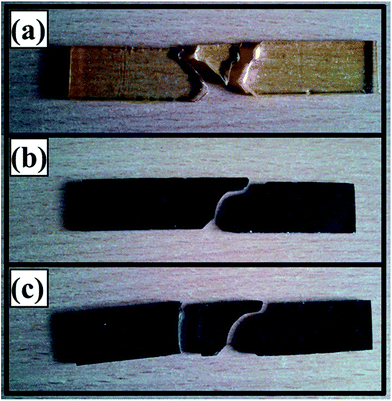
The fractured samples after Charpy impact testing are shown in Fig. 7. The monolithic epoxy split into three pieces as shown in Fig. 7(a). The smallest fragment was broken from the notch indicating a high level of stress concentration caused by the pointed notch. Therefore, when the hammer hit the specimen, the specimen broke into pieces next to notch. A shift in fracture behavior was observed in 0.1 wt% MH as shown in Fig. 7(b). The specimen broke into two fragments with the fracture path showing diversion which may be attributed to obstruction of the crack by the MLG. A relatively different fracture pattern was observed at 0.1 wt% MA specimen as shown in Fig. 7(c). Not only the fracture took place from the notch, but also the specimen got fractured away from the notch. It shows that there was very high stress concentration away from the notch. The notch is not supposed to generate such a high level of stress to cause fracture away from its tip. The only variable is the addition of organic solvent acetone which implies that acetone caused porosity, inhomogeneous distribution of MLG, and/or weakening of epoxy chains that generated sufficient amount of stress concentration to cause fracture away from notch tip. As surface energy is produced when fracture takes place, energy is required to produce fracture surfaces.39 Four fracture surfaces were produced in case of 0.1 wt% MA specimens which implies that more energy will be utilized than in case of 0.1 wt% MH specimens where two fracture surfaces were produced. However, the lower values of impact toughness in case of 0.1 wt% MA specimens indicate that stress concentration factor was dominant over surface energy factor.
In general, the mechanical properties of the nanocomposites with acetone were found lower than that without acetone. The acetone residues cause porosity in the samples which act as stress concentration sites and deteriorate the mechanical properties.28–30 The porosity caused by acetone can be viewed in optical micrographs. Fig. 8(a) shows optical micrograph of 1 wt% MLG–epoxy sample with acetone. Round porosity can be observed in the optical micrograph. The average pore diameter was up to 80 μm. The round porosity is caused by fluids as fluids exert uniform pressure in all sides. Therefore, this round porosity comes from air entrapment and evaporation of acetone residues. The porosity was also observed in samples without acetone as shown in Fig. 8(b). However, the porosity is non-circular which comes from the relative movement of resin and MLG during curing or restriction on pore shape by MLG.40 Loos et al.28 reported significant drop in Young's modulus, tensile strength and fracture strain as a result of residual acetone. The drop in mechanical properties was found directly related with the amount of acetone used.28 The traces of organic solvents influence cure kinetics and restrict cross-linking process.29 Hong and Wu30 mentioned that residues of organic solvents result in lower curing exotherm, reaction rate, initial curing rate, glass transition temperature (Tg), and reaction order. They also reported that organic solvents with higher boiling points have greater effect on cure kinetics and mechanical properties of epoxy.30
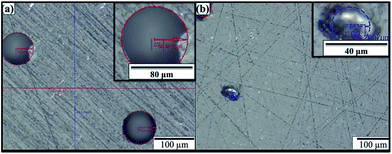 | ||
| Fig. 8 Optical micrographs of MLG–epoxy nanocomposites (a) with acetone (b) without acetone. The insets show porosity at higher magnification. | ||
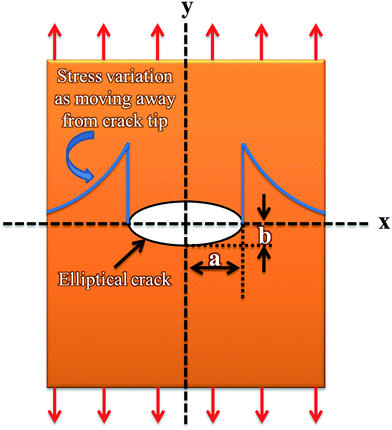
When a structural member contains a discontinuity, such as a hole, high localized stresses occur near the discontinuity as shown in Fig. 9. The maximum stress (σmax) at the ends of the hole is given by eqn (7).41
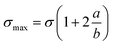 | (7) |
For a circular hole (a = b), σmax becomes three times of applied stress. The σmax further increases as the notch becomes elliptical and pointed. Therefore, a very narrow crack oriented normal to the tensile axis will result in a very high stress concentration. The effect of stress raiser is much more pronounced in a brittle material than in a ductile material. In a ductile material, plastic deformation occurs when the yield stress is exceeded at the point of maximum stress. Further increase in load produces a local increase in strain at the critically stressed region with little increase in stress. Because of strain hardening, the stress increases in regions adjacent to the stress raiser, until if the material is sufficiently ductile, the stress distribution becomes essentially uniform. Thus, a ductile material loaded statically will not develop the full theoretical stress-concentration factor. However, redistribution of stress will not occur to any extent in a brittle material. Therefore, a stress concentration of close to theoretical value will result in a brittle material. According to Griffith crack theory, discontinuities in brittle materials will significantly lower the mechanical properties.42 As epoxy has fragile structure, therefore stress concentration caused by retained acetone is pronounced and significantly deteriorates the mechanical properties of epoxy nanocomposites.
The dynamic mechanical properties of nanocomposites with 0.1 wt% MLG are shown in Fig. 10. Fig. 10(a) shows variation in tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ. The epoxy with acetone (EA) shows higher tan
δ. The epoxy with acetone (EA) shows higher tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ than that without acetone (E). The remnants of acetone significantly influenced the properties of non-reinforced epoxy. The 0.1 wt% of MLG in acetone (MA) showed little impact on tan
δ than that without acetone (E). The remnants of acetone significantly influenced the properties of non-reinforced epoxy. The 0.1 wt% of MLG in acetone (MA) showed little impact on tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ. It corroborates the trends observed for mechanical properties and optical micrographs.
δ. It corroborates the trends observed for mechanical properties and optical micrographs.
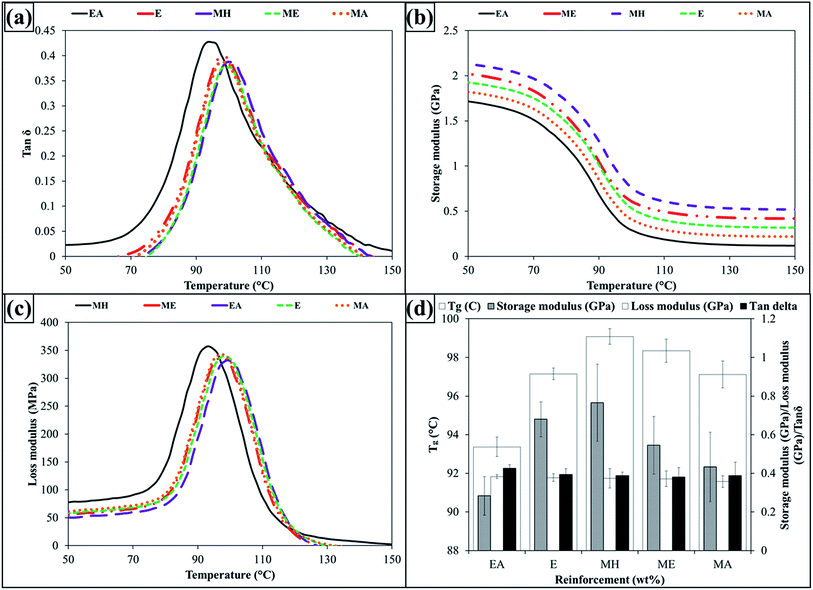 | ||
Fig. 10 (a) tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, (b) storage modulus, (c) Tg, and (d) values of storage modulus, loss modulus and tan δ, (b) storage modulus, (c) Tg, and (d) values of storage modulus, loss modulus and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ at Tg. δ at Tg. | ||
However, significant variation was observed in case of 0.1 wt% of MLG in epoxy (ME) and 0.1 wt% of MLG in hardener (MH) where relatively lower tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values were observed. The variation in tan
δ values were observed. The variation in tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ comes from storage and loss moduli as shown in Fig. 10(b) and (c). The increase in stiffness and restriction in the movement of polymer chains by MLG caused increase in storage modulus and decrease in loss factor in ME and MH. One indicator of the restriction in polymer chains is glass transition temperature (Tg) as shown in Fig. 10(d). An increase in Tg with MLG in hardener (MH) shows that MLG is relatively uniformly dispersed. When MLG is uniformly dispersed, the wrinkled texture and high surface area influence the maximum exothermic heat flow temperature by restricting polymer chain mobility that results in Tg rise.14 Fig. 10(d) also shows storage modulus, loss modulus, and tan
δ comes from storage and loss moduli as shown in Fig. 10(b) and (c). The increase in stiffness and restriction in the movement of polymer chains by MLG caused increase in storage modulus and decrease in loss factor in ME and MH. One indicator of the restriction in polymer chains is glass transition temperature (Tg) as shown in Fig. 10(d). An increase in Tg with MLG in hardener (MH) shows that MLG is relatively uniformly dispersed. When MLG is uniformly dispersed, the wrinkled texture and high surface area influence the maximum exothermic heat flow temperature by restricting polymer chain mobility that results in Tg rise.14 Fig. 10(d) also shows storage modulus, loss modulus, and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values at Tg. It can be observed that storage modulus increased while loss modulus and tan
δ values at Tg. It can be observed that storage modulus increased while loss modulus and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ decreased in case of MLG in epoxy and hardener compared with monolithic epoxy.
δ decreased in case of MLG in epoxy and hardener compared with monolithic epoxy.
The SEM images of nanocomposites are shown in Fig. 11. Fig. 11(a) and (b) show fractured surface of tensile specimen of monolithic epoxy. River markings can clearly be observed that indicate brittle fracture has taken place.14 It is because there are no crack bridging mechanisms available in monolithic epoxy. Therefore, once crack is initiated, it propagates beeline resulting in straight fracture paths. However, when reinforcement is introduced, fracture mode changes due to obstruction of the cracks by the reinforcement. This can be observed in Fig. 11(c) and (d) which show fractured surface of tensile specimen of 0.1 wt% MLG–epoxy nanocomposite with MLG dispersed in hardener (MH). No specific orientation of crack propagation was observed. It is because the MLG has the ability to prevent the advancement of cracks and cracks detour around the MLG to proceed.43 The SEM images of MA are shown in Fig. 11(e) and (f) which show that the cracks became round and parabolic in shape. In addition, porosity was observed as indicated by the arrows. This porosity may arise from the acetone. Therefore, the presence of acetone not only changed the fracture pattern but was also manifested in mechanical properties.
The relationship between dispersion state and nature of crack advancement is schematically shown in Fig. 11(g) and (h). Fig. 11(g) is a schematic of poorly dispersed agglomerated graphene in the epoxy matrix. As graphene sheets have stress concentration factors associated with them, (micro-) cracks are generated around the graphene agglomerates. These (micro-) cracks may propagate under the application of external load and may lead to fracture. If there is a pre-existing crack in the matrix, it will propagate when load is applied. If the crack faces the agglomerate, it will either be restrained by the agglomerate or detour/bifurcate to circumvent the agglomerate in case of higher loads. However, as graphene is present in the form of agglomerates, a major portion of the epoxy matrix is not reinforced at all. Therefore, a crack can easily propagate through the brittle epoxy until fracture occurs. This is possibly the reason why poorly dispersed graphene was not found to be efficient in improving the fracture toughness of epoxy.14 On the contrary, if graphene is uniformly dispersed, it would be difficult for the crack to move. Fig. 11(h) shows a schematic diagram for an ideal situation in which graphene of nearly same dimensions is homogeneously dispersed into an epoxy matrix. In this case, as sheet size is relatively smaller than that of graphene agglomerate, the stress concentration factor associated with them is smaller and there is almost no (micro-) cracking around individual graphene sheets. If there is a pre-existing crack in the matrix and it starts propagating under the influence of external load, it has to cross graphene sheets at each step. If the external load is high enough, each crack will split into multiple sub-cracks. There is required energy at each division and sub-division of the crack to generate new surfaces. Therefore, extensive energy will be dissipated before the crack system advances to reach a critical length that causes fracture. This will significantly improve the fracture toughness of the epoxy. Therefore, uniformly dispersed graphene is preferred to improve the fracture toughness of the epoxy–graphene nanocomposites.
Conclusions
Nanocomposites of three different types were successfully produced. The MLG and dispersion medium significantly influenced the behaviour of nanocomposites. The maximum increase in tensile and flexural properties, fracture toughness, and microhardness were observed at 0.3 wt% of MLG (MH). The Young's modulus increased from 610 MPa to 758 MPa (24% increase) and the tensile strength increased from 46 MPa to 60 MPa (32% increase). The fracture toughness (K1C) increased from 0.8 MPa m1/2 to 1.1 MPa m1/2 (29% increase) and the Charpy impact toughness increased from 0.85 kJ m−2 to 1.61 kJ m−2 (89% increase). The MLG has the ability to increase the storage modulus and glass transition temperature (Tg) by increasing the stiffness of nanocomposites and restricting the polymer chains. SEM images showed that MLG can obstruct the advancing cracks and significantly influence fracture mode. The MLG dispersed in low viscosity hardener without the use of organic solvent as dispersion medium can significantly improve the mechanical properties. The organic solvent as dispersion medium, if not completely removed, causes porosity which is detrimental to mechanical properties. However, it should be noted that it is not the use of acetone that lowered the mechanical properties of nanocomposites. In fact, it is the residuum of acetone that deteriorated the mechanical properties of nanocomposites. The results may be different if organic solvent is completely removed. Nevertheless, some residues of solvent are expected in fast-curing epoxy resin. Therefore, if hardener has low viscosity and resin is fast-curing, MLG can be dispersed in hardener for improved mechanical properties.Acknowledgements
The authors would like to thank the Department of Mechanical and Construction Engineering, Northumbria University, UK for the provision of research facilities, without which the analysis of relevant data was not possible.References
- J. Wei, R. Atif, T. Vo and F. Inam, J. Nanomater., 2015, 2015, 1–12 Search PubMed.
- M. S. Saharudin, R. Atif, I. Shyha and F. Inam, RSC Adv., 2016, 6, 1076–1089 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science., 2004, 306, 666–669 CrossRef CAS PubMed.
- P. Pokharel, Q.-T. Truong and D. S. Lee, Composites, Part B, 2014, 64, 187–193 CrossRef CAS.
- M. Tian, L. Qu, X. Zhang, K. Zhang, S. Zhu, X. Guo, G. Han, X. Tang and Y. Sun, Carbohydr. Polym., 2014, 111, 456–462 CrossRef CAS PubMed.
- R. Atif, J. Wei, I. Shyha and F. Inam, RSC Adv., 2016, 6, 1351–1359 RSC.
- R. Bkakri, A. Sayari, E. Shalaan, S. Wageh, A. A. Al-Ghamdi and A. Bouazizi, Superlattices Microstruct., 2015, 76, 461–471 CrossRef.
- Y. Lian, F. He, H. Wang and F. Tong, Biosens. Bioelectron., 2015, 65, 314–319 CrossRef CAS PubMed.
- Z. Abdin, M. A. Alim, R. Saidur, M. R. Islam, W. Rashmi, S. Mekhilef and A. Wadi, Renewable Sustainable Energy Rev., 2013, 26, 837–852 CrossRef CAS.
- W. Sun, R. Hu, H. Liu, M. Zeng, L. Yang, H. Wang and M. Zhu, J. Power Sources, 2014, 268, 610–618 CrossRef CAS.
- A. A. Azeez, K. Y. Rhee, S. J. Park and D. Hui, Composites, Part B, 2013, 45, 308–320 CrossRef CAS.
- A. Aziz, H. N. Lim, S. H. Girei, M. H. Yaacob, M. A. Mahdi, N. M. Huang and A. Pandikumar, Sens. Actuators, B, 2015, 206, 119–125 CrossRef.
- N. Agnihotri, A. D. Chowdhury and A. De, Biosens. Bioelectron., 2015, 63, 212–217 CrossRef CAS PubMed.
- L.-C. Tang, Y.-J. Wan, D. Yan, Y.-B. Pei, L. Zhao, Y.-B. Li, L.-B. Wu, J.-X. Jiang and G.-Q. Lai, Carbon, 2013, 60, 16–27 CrossRef CAS.
- S.-Y. Lee, M.-H. Chong, M. Park, H.-Y. Kim and S.-J. Park, Carbon Lett., 2014, 15, 67–70 CrossRef.
- I. Zaman, B. Manshoor, A. Khalid, Q. Meng and S. Araby, J. Mater. Sci., 2014, 49, 5856–5865 CrossRef CAS.
- J. Wei, T. Vo and F. Inam, RSC Adv., 2015, 5, 73510–73524 RSC.
- S. Chandrasekaran, C. Seidel and K. Schulte, Eur. Polym. J., 2013, 49, 3878–3888 CrossRef CAS.
- T. Kuila, S. Bose, A. K. Mishra, P. Khanra, N. H. Kim and J. H. Lee, Prog. Mater. Sci., 2012, 57, 1061–1105 CrossRef CAS.
- J. Karger-Kocsis, H. Mahmood and A. Pegoretti, Prog. Mater. Sci., 2015, 73, 1–43 CrossRef CAS.
- Y. Zhang, Y. Wang, J. Yu, L. Chen, J. Zhu and Z. Hu, Polymer, 2014, 55, 4990–5000 CrossRef CAS.
- J. Jang, J. Colloid Interface Sci., 2014, 424, 62–66 CrossRef CAS PubMed.
- Z. A. Ghaleb, M. Mariatti and Z. M. Ariff, Composites, Part A, 2014, 58, 77–83 CrossRef CAS.
- J. a. King, D. R. Klimek, I. Miskioglu and G. M. Odegard, J. Appl. Polym. Sci., 2013, 128, 4217–4223 CrossRef CAS.
- X. Wang, L. Song, W. Pornwannchai, Y. Hu and B. Kandola, Composites, Part A, 2013, 53, 88–96 CrossRef CAS.
- W. P. Serena Saw and M. Mariatti, J. Mater. Sci.: Mater. Electron., 2011, 23, 817–824 CrossRef.
- I. Zaman, H.-C. Kuan, Q. Meng, A. Michelmore, N. Kawashima, T. Pitt, L. Zhang, S. Gouda, L. Luong and J. Ma, Adv. Funct. Mater., 2012, 22, 2735–2743 CrossRef CAS.
- M. R. Loos, L. A. F. Coelho, S. H. Pezzin and S. C. Amico, Polimeros, 2008, 18, 76–80 CrossRef CAS.
- K. Lau, M. Lu, H. Cheung, F. Sheng and H. Li, Compos. Sci. Technol., 2005, 65, 719–725 CrossRef CAS.
- S. Hong and C. Wu, Thermochim. Acta, 1998, 316, 167–175 CrossRef CAS.
- T. Morishita, Carbon, 2011, 49, 5185–5195 CrossRef CAS.
- J. Yanmei, Mater. Chem. Phys., 2012, 135, 948–956 CrossRef.
- G. Farzi, Polymer, 2009, 50, 5901–5908 CrossRef CAS.
- S.-Z. Kang, Colloids Surf., A, 2011, 384, 363–367 CrossRef CAS.
- B. Munkhbayar, Chem. Eng. Process., 2012, 61, 36–41 CrossRef CAS.
- Z. Fan, F. Gong, S. T. Nguyen and H. M. Duong, Carbon, 2015, 81, 396–404 CrossRef CAS.
- M. A. Rafiee, J. Rafiee, Z. Wang, H. Song, Z. Yu and N. Koratkar, ACS Nano, 2009, 3, 3884–3890 CrossRef CAS PubMed.
- Y.-J. Wan, L.-C. Tang, L.-X. Gong, D. Yan, Y.-B. Li, L.-B. Wu, J.-X. Jiang and G.-Q. Lai, Carbon, 2014, 69, 467–480 CrossRef CAS.
- B. Pramanik, T. Tadepalli and P. R. Mantena, Materials, 2012, 5, 922–936 CrossRef.
- D. R. Bortz, E. G. Heras and I. Martin-Gullon, Macromolecules, 2012, 45, 238–245 CrossRef CAS.
- G. E. Dieter, Mechanical Metallurgy, McGraw-Hill, SI Metric, 1988 Search PubMed.
- A. A. Griffith, Philos. Trans. R. Soc. London, Ser. A, 1921, 221, 163–198 CrossRef.
- H. Feng, X. Wang and D. Wu, Ind. Eng. Chem. Res., 2013, 52, 10160–10171 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |