Eco-friendly photolithography using water-developable pure silk fibroin†
Abstract
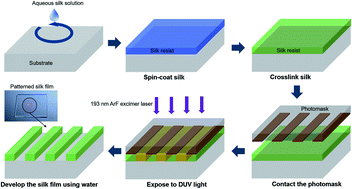
We report that pure silk fibroin can be a green and biofunctional photoresist for deep ultraviolet photolithography. All processes are entirely water-based, from resist solvent to resist removal, and rely on the phototendering effect that decreases the crystallinity of silk fibroin films by DUV exposure. Additionally, the potential decrease in activity of bio-dopants due to high-energy irradiation is irrelevant to our positive-tone lithographic method.

 Please wait while we load your content...
Please wait while we load your content...