Gold nanoparticles encapsulated in hierarchical porous polycarbazole: preparation and application in catalytic reduction†
Abstract
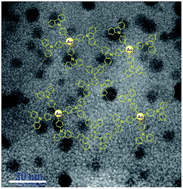
Porous organic polymers possessing high surface area and permanent porosity are emerging as a new kind of catalyst support. In this article, surface-functionalized gold nanoparticles (AuNPs) were presynthesized and further subjected to the FeCl3-promoted oxidative coupling copolymerization with 1,3,5-tri(9H-carbazol-9-yl)benzene to afford AuNPs encapsulated in porous polycarbazole (AuNPs@CPOP) under mild conditions. Through this preparative approach, the AuNPs do not occupy the cavities of the polymer, but instead are surrounded by grown porous polymer. Furthermore, transmission electron microscopy shows that the AuNPs are homogeneously embedded in the polymeric support with a narrow size distribution. The nitrogen sorption measurements show that AuNPs@CPOP possesses high porosity, the pore size distributions are hierarchical with micro-, meso-, and macropore size. The catalytic activities of the final porous composite materials are studied by the reduction of 4-nitrophenol to 4-aminophenol. The catalytic activity of AuNPs@CPOP is found to be outstanding with an activity factor of up to 17.57 s−1 g−1. The conjugated polycarbazole-encapsulated AuNPs have potential application in the fields of environmental chemistry and catalysis.

 Please wait while we load your content...
Please wait while we load your content...