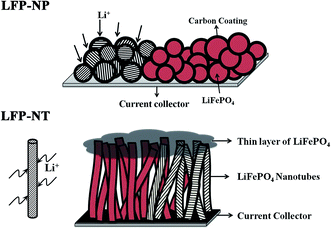
High-rate capability of bamboo-like single crystalline LiFePO4 nanotubes with an easy access to b-axis 1D channels of olivine structure
M. Viji,
Pravati Swain,
Pavana S. V. Mocherla and
C. Sudakar*
Multifunctional Materials Laboratory, Department of Physics, Indian Institute of Technology Madras, Chennai-600036, India. E-mail: csudakar@iitm.ac.in; Tel: +91-44-2257-4895
First published on 13th April 2016
Abstract
Surfactant aided sol–gel method is used to fabricate highly crystalline carbon-coated LiFePO4 (LFP) in two contrasting microstructural forms: (i) nanoparticulate crystallites (LFP-NP), and (ii). bamboo-like single crystalline nanotubes (LFP-NT) obtained using template-assisted method. LFP-NT exhibit a high specific capacity (∼165 mA h g−1 at 1C-rate) and superior rate capability with reversible capacity of ∼100 mA h g−1 (∼60 mA h g−1) at a current rate of 10C (25C) with almost 100% capacity retention after 1000 cycles, whereas, LFP-NP exhibit poor capacity retention even at low C-rates (<10 mA h g−1 at 5C-rate). Although shortened pathways for Li transport exist in both the microstructures, the key point to achieve high-rate capability in LFP system is to facilitate easy access to the entry points of 1D channels running along b-axis of olivine structure. The curved cylindrical surfaces of bamboo-like nanotubes have a large proportion of these entry points. The nanoparticles have limited access to these entry points due to agglomeration, therefore exhibiting poor capacity retention at high C-rates. Fabricating LFP with microstructural-openness with a large number of accessible 1D-channel entry points on the surface of LFP is crucial for achieving high-rate capability cathode.
I. Introduction
Lithium iron phosphate, LiFePO4 (LFP) with olivine-type crystal structure possesses one dimensional (1D) diffusion paths for lithium (Li) intercalation. Despite the existence of 1D Li diffusion paths in olivine structure, unlike the layered cathode materials with 2D Li-diffusion (e.g. LiCoO2) and spinel compounds with 3D-Li diffusion (e.g. LiMn2O4), LiFePO4 has drawn significant attention as a promising cathode for Li-ion battery (LIB) applications for its high theoretical capacity (170 mA h g−1), operating voltage (3.4 V vs. Li+/Li), safety and environmental benignity, and low cost.1 Inspite of these advantages, LFP compound suffers from low intrinsic electronic conductivity (10−8 to 10−10 S cm−1) and low diffusion coefficient (10−10 to 10−16 cm2 s−1), due to which commercializing LFP as a viable high-rate capability cathode material is a big challenge.2,3 Various modifications to the structure and microstructure have been attempted to address sluggish charge and mass transport. These include cation doping,2,4–6 carbon coating,7 and reduced particle size.8Common strategies to improve high energy density and faster Li intercalation/de-intercalation include reducing the effective diffusion length by reducing the physical size of the particles to nanometer scale.9 Self-assembled single crystalline LiFePO4 nanowires were shown to exhibit reversible capacity of ∼110 mA h g−1 at 30C-rate with highly stable capacity retention.10 Various synthesis processes were proposed to prepare nanometer-sized LiFePO4 with different morphologies as the microstructural features of anodes and cathodes are being realized as an important control parameter for achieving good electrochemical performance.11–16 Nanowires prepared by electrospinning process with efficient conductive carbon network were shown to exhibit very good rate performance and cyclic capability.7 Good performance in the electrochemical properties has also been shown in unique one dimensional anode nanostructures for lithium ion battery applications by Y. C. Kang's group.17,18 Single crystalline LiFePO4 nanosheets with highly exposed facets were shown to retain significant capacities at high C-rates.19,20 The time for Li-ion to diffuse along [010] direction of olivine compound nanosheets, with sheet thickness along [010], were shown to be five orders of magnitude lower than the bulk values.21 All these works suggest that a preferable exposure of Li-ion in solution with an easy access to b-axis in LFP is the best way to achieve high-rate capability. However, large variation in the reported rate capability at these nanometer scale indicates that there are other factors limiting the effective electron and Li-ion transport through the nanoparticulate layers. The rate of diffusion during the intercalation and de-intercalation depends on the diffusion coefficient of the materials, typically 10−10 to 10−16 cm2 s−1 for LiFePO4. Many a time the diffusion reaction is limited by the accessibility of Li ions at the surface of LiFePO4 due to tightly packed nanoparticulate agglomerates.
In this report, we show that bamboo-like vertically standing LFP nanotube bundles enable a better accessibility for Li-ion movement along 1D-channels in b-axis of olivine structure. Detailed structural and electrochemical properties were carried out on (i) LFP in the porous nanoparticulate agglomerates with limited access to the 1D-channels and (ii) the bundles of bamboo-like vertically standing nanotubes with an easy access to the channels along b-axis, both prepared by same sol–gel precursor. The nanotube bundles with entry points to the diffusion channels lying on the cylindrical surface area allow large number of Li-ions to intercalate/de-intercalate easily, thereby enabling high rate capability of 50C. Significant capacity at high C-rates (∼100 mA h g−1 at 10C and ∼60 mA h g−1 at 25C) and cyclic stability over 1000 cycles are exhibited by these nanotube LFP bundles.
II. Experimental
LiFePO4 nanoparticles (LFP-NP) were synthesized via a surfactant based sol–gel method.19 Separate solutions of lithium acetate dihydrate (CH3CO2Li·2H2O, 99% from Loba Chemie), Ferrous chloride tetrahydrate (FeCl2·4H2O, 98% from Alfa Aesar), and phosphorous pentoxide (P2O5, 97% from Merck) of 1 M each were prepared in ethanol (99.9% from SRL Pvt. Ltd.) medium. The FeCl2 and P2O5 solutions were mixed in stoichiometric ratio and stirred for 3 h followed by addition of Li precursor. This stirring was continued for 3 h after this addition and finally 1 M lauric acid [CH3(CH)2COOH, 99% from Loba chemie] was added as the surfactant to control the particle size as well as a source of carbon coating on the LFP particles. Stirring was carried out in an inert N2 atmosphere (99.9%) to prevent the oxidation of Fe2+ to Fe3+. This solution mixture was again stirred for 4 h. This final precursor solution is dried to obtain precursor powder for LFP-NP and used for immersing 10 μm thick polycarbonate membrane with ion track-etched pores of 400 nm diameter with a pore density of ∼1.3 × 108 pores per cm2 (Isopore™ membrane filters, Merck Millipore Ltd.) for 24 h to obtain LFP nanotube (LFP-NT). Dried precursor powders were annealed at 550 °C in H2 + N2 (5% + 95%) atmosphere for 5 h in order to get carbon-coated LiFePO4 nanoparticle sample. The polycarbonate membrane filled with precursor solution into the pores was laid on 15 μm thick aluminum foil (MTI Corp. Inc., Richmond, CA, USA), dried on a hot plate maintained at 80 °C for 1 h. After the membrane adheres to the Al foil the temperature is slowly increased to 200 °C and kept at that temperature for 20 min. Then the final annealing is carried out at 650 °C for 12 h with heating rate 2 °C min−1 in H2 + N2 (5% + 95%) atmosphere. This process gives LFP nanotube bundles which are referred to as LFP-NT.Phase purity of the LFP-NP and LFP-NT samples was studied using X-ray power diffractometer (X'pert-Pro, Panalytical) with a 1.2 kW Cu-Kα, λ = 1.5406 Å source. The microstructure of LFP-NP and LFP-NT samples were studied by scanning electron microscopy (SEM: FEI Quanta FEG 400) and the high resolution transmission electron microscopy (FEI Tecnai T20 operating at 200 kV). The carbon coating on the LFP-NP and LFP-NT samples were investigated by the Raman spectroscopy using a Horiba Jobin-Yvon (HR 800 UV) spectrometer using 488 nm (from Ar-ion laser) excitation wavelength.
The electrochemical properties of LFP-NT and LFP-NP samples were evaluated using a CR2032 type two electrode coin-type cell in the half-cell configuration. The chemicals and the CR2032 coin cell assemblies used in the experiment are all purchased from MTI corp. Inc., Richmond, CA, USA. The cathode using LFP-NP, was fabricated by mixing 80% active material with 10% acetylene black and 10% PVDF binder. N-Methyl-2-pyrrolidone (NMP) was used as solvent to prepare the slurry for cathode material coating on the current collector. The slurry was coated over a 15 μm thick Al foil. The thick cathode layer coating is dried under the vacuum (∼10−2 bar) at 100 °C for 12 h. For the LFP-NT the coating obtained on the Al foil is directly employed as cathode without any further processing. In the half cell fabrication lithium metal (99.9% from Alfa Aesar) is used as anode and polypropylene membrane is used as separator. The electrolyte used in the button cell is 1 M LiPF6 in ethylene carbonate (EC), di-methyl carbonate (DMC) and di-ethyl carbonate (DEC) organic solvents in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (vol%). The LFP-coated Al electrode is cut into circular sheets (16 mm diameter). The mass loading of LiFePO4 on the 16 mm diameter Al foil is ∼5.6 mg for LFP-NP and ∼0.56 mg for LFP-NT. The coin cell was 20 mm in diameter and 3.2 mm thick (CR2032) and the entire cell was assembled in argon filled inert glove box maintained with water and oxygen below 0.1 ppm level. The electrochemical performance of the cathode material was evaluated by galvanostatically cycling at different rates (0.1C to 50C) over the potential range 2 to 4.0 V versus Li/Li+ using VSP150 (Biologic) multichannel electrochemical workstation. Cyclic voltammetry (CV) was performed at different scan rates (0.05 mV s−1 to 1 mV s−1) between 2 and 4.5 V. Electrochemical impedance was also carried out on coin cells using VSP150 (Biologic) electrochemical workstation in the frequency range of 10 mHz to 1 MHz with a sinusoidal excitation voltage of 10 mV.
1 (vol%). The LFP-coated Al electrode is cut into circular sheets (16 mm diameter). The mass loading of LiFePO4 on the 16 mm diameter Al foil is ∼5.6 mg for LFP-NP and ∼0.56 mg for LFP-NT. The coin cell was 20 mm in diameter and 3.2 mm thick (CR2032) and the entire cell was assembled in argon filled inert glove box maintained with water and oxygen below 0.1 ppm level. The electrochemical performance of the cathode material was evaluated by galvanostatically cycling at different rates (0.1C to 50C) over the potential range 2 to 4.0 V versus Li/Li+ using VSP150 (Biologic) multichannel electrochemical workstation. Cyclic voltammetry (CV) was performed at different scan rates (0.05 mV s−1 to 1 mV s−1) between 2 and 4.5 V. Electrochemical impedance was also carried out on coin cells using VSP150 (Biologic) electrochemical workstation in the frequency range of 10 mHz to 1 MHz with a sinusoidal excitation voltage of 10 mV.
III. Results and discussion
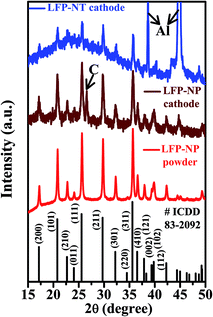
The X-ray diffraction (XRD) patterns of the LFP-NP powder, LFP-NP cathode and LFP-NT cathode are shown in Fig. 1 along with a standard powder pattern for orthorhombic olivine structure of LiFePO4 (ICDD file 01-083-2092; Pnma space group). The phase in the nanotube samples is found to be similar to that of powder samples. The XRD peaks of LFP-NT are lesser intense due to thinner nanotube cathode layers (∼10 μm) on Al foil and due to the low mass loading of LFP-NT. Such difference in the intensity is also noted between the LFP-NP powder and LFP-NP cathode. Strong peaks at 2θ ∼ 39° and 45° are from Al which is used as current collector for cathode material. However, the intense peaks (200), (101), (111), (211), and (311) in LFP-NT match well with the corresponding LFP standard peaks. The powder samples have lattice parameters of a = 10.32 Å, b = 6.0017 Å, c = 4.689 Å as obtained from the Rietveld refinement of these XRD patterns (not shown).19 The crystallite size for LFP-NP as estimated from the refinement is ∼40 nm. The LFP-NT samples are also found to have similar crystallite size.Raman spectroscopy using a 488 nm excitation from an Ar-ion laser of (i) LFP-NP powder, (ii) LFP-NP powder fabricated on the Al-foil in the electrode form (LFP-NP cathode) after mixing with additives including acetylene black (conductive carbon source), and (iii) the LFP-NT directly fabricated on Al-foil (LFP-NT cathode) are shown in Fig. 2. The Raman bands are deconvoluted into four Gaussian peaks centered ∼1180, 1343, 1490 and 1591 cm−1.19,22–24 The two bands at ∼1340 and ∼1590 cm−1 corresponds to the D (disorder) and G (graphitic) bands of carbon.22,24 The G band ∼1590 cm−1 corresponds to the first order scattering of E2g modes, i.e. bond stretching of all pairs of sp2 atoms in both rings and chains.25 The D-band is related to the defects in the graphitic carbon such as bond-angle disorder, bond-length disorder, vacancies, edge defects, etc.25 Therefore these bands are assigned to sp2 graphite like structure. Two additional bands ∼1200 cm−1 and ∼1500 cm−1 are assigned to short-range vibrations of sp3-coordinated carbons. The differences in the widths and intensities of D and G bands in these samples are attributed to the different carbon phases. The D/G ratios and sp2/sp3 ratios estimated for the three samples are listed in the Table 1. The values give an idea on the degree of carbon disorder (i.e. D/G ratio) and the content of sp2 and sp3-like disordered carbon in the sample. The D/G ratio is found to be small (∼1.71) for LFP-NT (on Al foil) compared to the LFP-NP (∼2.65) or LFP-NP electrode comprising of acetylene black fabricated on Al foil (∼1.9). Also the sp2/sp3 ratio for LFP-NT is large compared to the LFP-NP samples indicating a better conductive carbon coating. These differences in the Raman spectral signatures indicate a better quality of carbon coating on the LFP-NT surface, which could be arising from the residual carbon due to the burning the polycarbonate membrane. Thus, the quality of the carbon inferred from the D/G ratio and sp2/sp3 ratio also indicates a superior electrochemical performance of the LFP-NT cathode compared to the LFP-NP cathode.
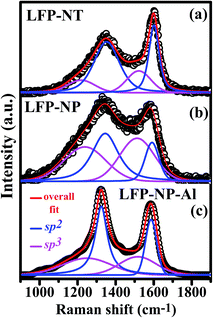 | ||
| Fig. 2 Raman spectra of carbon coating from LFP-NP (powder), LFP-NT (on Al foil) and LFP-NP-Al (LFP-NP electrode fabricated on Al) obtained using blue laser of wavelength 488 nm. | ||
| Sample ID | Integrated D/G | Integrated sp2/sp3 (d-C) |
|---|---|---|
| LFP-NP (powder) | 2.65 | 0.73 |
| LFP-NT (on Al) | 1.71 | 2.72 |
| LFP-NP-Al (electrode fabricated on Al) | 1.9 | 1.19 |
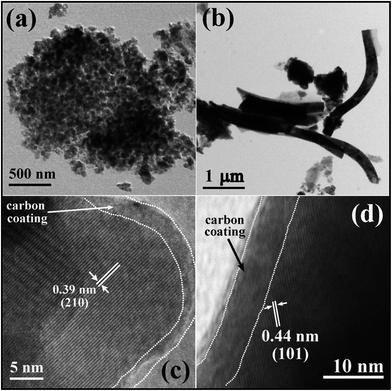
The microstructures of LFP-NP and LFP-NT were investigated from the field emission scanning electron microscopy (FESEM) images (see Fig. 3) and transmission electron microscopy (TEM) images (see Fig. 4 and 5). The FESEM image of LFP-NT sample shows of vertically standing bunch of nanotubes with bamboo-like microstructure (Fig. 3a–c). The top surface of nanotube-bunches are covered with a thin layer of LFP with sparsely dispersed nanoparticles which could be resulting from a thin layer of precursor solution left on the surface of polycarbonate membrane. Unlike the LFP-NP samples in which the LFP nanoparticles are agglomerated compactly (Fig. 3d and 4a) the LFP-NT samples have well-dispersed nanotubes so that Li-ions from the electrolyte can easily permeate and reach individual nanotube surfaces (Fig. 3b and c). The LFP-NP have a thin layer of carbon coating as depicted in Fig. 4c. The nanotubes and the thin surface layer of LFP covered with a carbon coating in LFP-NT also improve the electronic conductivity of cathode and reduce the degradation of active material due to direct contact with the electrolyte (Fig. 4d). The carbon coating in LFP-NT is thicker (∼5 to 10 nm) than that seen on the LFP-NP surfaces (∼2–5 nm). The nanotubes in LFP-NT samples have an average diameter ∼250 nm and length ∼5 to 10 μm. These values are smaller than the size and length of pores present in the membrane. This difference in the dimension of nanotubes and the size of membrane pores results due to the shrinkage of filled material during drying and annealing process. The high resolution TEM images show the single crystalline nature of the nanotubes (Fig. 5a and b). The nanotubes also show assembly of nanoparticles on the surface which are highly oriented as shown in HRTEM image of Fig. 5c. The nanoparticles, despite on the surface, exhibit the same lattice fringes as that of the interior of the nanotubes indicating single crystalline nature with same structural features. The SAD patterns obtained from three different regions (marked 1, 2, 3) in Fig. 5b show same diffraction pattern confirming the highly oriented nature of nanoparticle assemblies. Interestingly, we find that the b-axis along which constitute the Li-ion diffusion channels, are mostly inclined to the cylindrical axis or perpendicular to the cylindrical surface of nanotubes. These microstructural details indicate that the entry points to the 1D-channels along the b-axis are mostly found on the cylindrical surface for the nanotube microstructure.
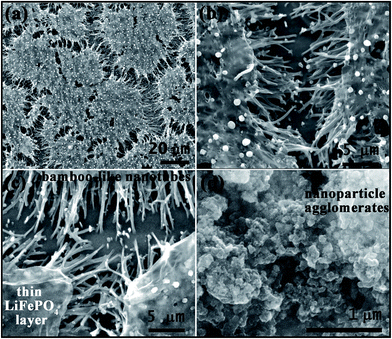 | ||
| Fig. 3 SEM images of LFP nanostructures cathode coated on Al foil. (a) to (c) correspond to LFP-NT at different magnifications and (d) shows the surface morphology of LFP-NP. | ||
The electrochemical property of widely different microstructures LFP-NP and LFP-NT is studied. Fig. 6 shows the galvanostatic charging and discharging voltage curves (2–4 V vs. Li/Li+) of LFP-NP and LFP-NT cathode layers. The measurements were carried out over a wide range of C-rates (0.05C to 50C). LFP-NP and LFP-NT show a flat profile ∼3.35 V and ∼3.43 V for 0.1C rate for a wide range with a discharging capacity of 130 mA h g−1 and 180 mA h g−1 respectively. The flat potential represent the extraction and insertion of Li-ions by two-phase mechanism.26 LFP-NP samples showed a significant drop in capacity, 65 mA h g−1 at 2C (and <10 mA h g−1 at 5C rate), with largely sloping potential decrease. On the other hand LFP-NT samples show large capacity (∼125 mA h g−1) at these C-rates. Interestingly, LFP-NT cathode exhibited a capacity of 25 mA h g−1 even at 50C-rate showing its high rate capability during charging–discharging cycles. The electrode potential of ∼3 V at 50C-rate further signifies a lower internal resistance in LFP-NT compared to LFP-NP for which the electrode potential is well below 3 V even at a current rate of 2C. The discharge performances of LFP-NP and LFP-NT tested at different C-rates as a function of cycle number are shown in Fig. 7. The constant charging–discharging capacity of LFP-NT over several cycles compared to LFP-NP, for which slight fluctuation in the capacity is noted, indicates the stability of LFP nanotube cathode material. This non-fading behavior with 100% columbic efficiency (CE) shows the stability of the material. After several charging–discharging cycles at various current rates the material retains the initial capacity 150 mA h g−1 at 1C-rate with a columbic efficiency of 100% (Fig. 7b). The capacity retention measurement for LFP-NT measured at a current rate of 10C showed a reduction in the capacity from 90 mA h g−1 to 50 mA h g−1 after 1000 cycles of charging–discharging (Fig. 8). This gradual decrease in capacity with the number of charging–discharging cycles at such high current rate could only be due to the limited supply of electrons to the active components in the cathode. This could arise due to the voltage polarization as well as an increase in internal impedance of the cell. Slight oscillations observed in the charging–discharging capacity in Fig. 8 are due to the variation of room temperature during the day and night time in the lab. However, it is interesting to see that the capacity reverts to the value 146 mA h g−1 when measured at 1C rate after 1000 charging–discharging cycles. The columbic efficiency (CE) of 100% over 1000 cycles also signifies excellent capacity retention of LFP-NT cathode material. In contrast, LFP-NP exhibit poor rate capability and capacity retention at high C-rates though both LFP-NP and LFP-NT samples were prepared from the same precursor solution, showing the superiority of the nanotube-based cathode.
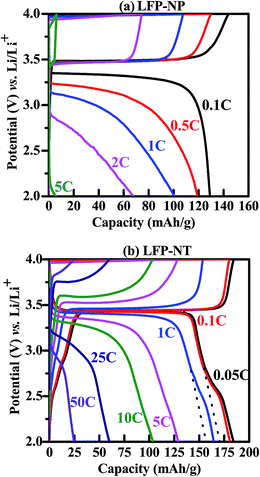 | ||
| Fig. 6 Galvanostatic charge–discharge curves measured at different C-rates (0.05C to 50C) vs. Li/Li+ for (a) LFP-NP and (b) LFP-NT. | ||
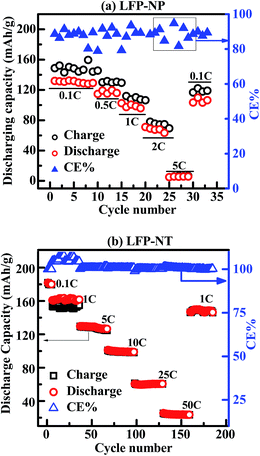 | ||
| Fig. 7 Discharge capacity (vs. Li/Li+) plotted vs. number of discharging cycles at different C-rates for (a) LFP-NP and (b) LFP-NT. | ||
 | ||
| Fig. 8 Capacity retention of LFP-NT measured at 10C rate for 1000 cycles. Note the capacity at 1C rate after 1000 cycles is close to the values obtained on fresh samples. | ||
It is also interesting to note that the discharging capacity in LFP-NP is lower than charging capacity for all the C-rates due to the characteristic of the material. However, the LFP-NT showed slightly over 100% columbic efficiency (CE) (by ∼1 to 2%) for most of the C-rates, which could be due to a reversible redox reaction between Li+ and the carbon coatings on LFP-NT. Similar behavior has been reported by Hu et al.27 with a specific capacity much higher than LFP phase. For 1C rate, during initial cycles of charging–discharging, the increase in CE was about 4 to 6%. We believe that this higher CE is due to an unidentified secondary phase. This secondary phase is clearly visible from the step in the charging–discharging cycles of LFP-NT for 0.05C and 1C rate at ∼2.5 V. The reversibility of Li intercalation/deintercalation in this particular secondary phase seems to be highly C-rate dependent. At low C-rate (0.05C and 0.1C) the Li intercalation/deintercalation is completely reversible and therefore the CE is ∼100%. For C-rates > 1C, this secondary phase becomes inactive and ∼100% CE is mainly due to the reversible electrochemical response of LFP-NT. But at 1C rate the secondary phase takes Li during discharging cycles, however does not leave the Li during charging cycles making the CE look >100%. This phase could either be an amorphous Li deficient Fe2O3 or a secondary phase arising due to the Al reacting with LFP at the current collector-LFP interface (due to 650 °C annealing). This secondary phase takes excess Li till it gets saturated. After several cycles of charging–discharging at various C-rates we find this irreversible Li intake is suppressed and the charging–discharging at 1C rate takes place in a reversible way (Fig. 7b). Thus we believe the slight increase in CE for LFP-NT over 100% is due to the carbon layers and CE over 4 to 6% during the initial stages of charging–discharging cycles is due to an unidentified secondary phase.
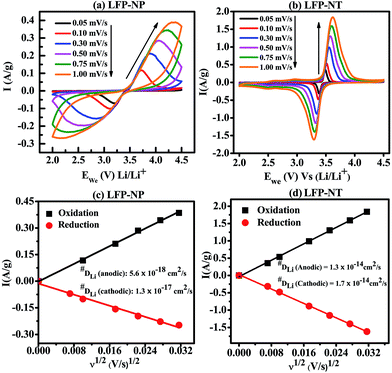
The diffusion coefficient of Li in LFP-NP and LFP-NT are determined from the cyclic voltammetric (CV) profiles (Fig. 9). The CV were measured with a linear potential sweep, using various scan rates from 0.05 to 1 mV s−1, applied to the electrode in the scan range of 2.0 to 4.5 V vs. Li/Li+. Both LFP-NP and LFP-NT electrodes show a pair of redox peaks, anodic peak during charging and cathodic peak during discharging, which is a characteristic of two-phase reaction occurring in LiFePO4. For both cathodes, the midpoint of anodic and cathodic peaks is ∼3.4 V, which is equivalent to the open-circuit voltage of the electrodes. The increase in peak separation with increasing scan rate is much higher for LFP-NP than the LFP-NT electrodes. At a scan rate 0.3 mV s−1 the oxidation and reduction peaks appear at ∼3.55 V and ∼3.34 V respectively for LFP-NT with a peak separation of 0.2 V whereas the separation is ∼1 V for LFP-NP with oxidation and reduction peak appearing ∼3.89 V at ∼2.84 V, respectively. However in both the cases, current-potential varies linearly during the initial increase in current and overlap regardless of the scan rate at the beginning of charging and discharging. The conspicuous difference between the LFP-NT and LFP-NP CV profiles is seen with the former exhibiting much sharper redox peaks than the latter. Large voltage hysteresis in LFP-NP is due to the poor diffusion arising in nanoparticle samples. The lithium ion diffusion coefficient can be calculated from the slope of ip/m vs. v0.5 plot using Randles–Sevcik equation,
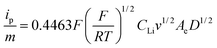 | (1) |
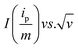 is shown in Fig. 9c and d for LFP-NP and LFP-NT, respectively. The calculated anodic and cathodic diffusion coefficients of Li for LFP-NP, using Brunauer–Emmett–Teller (BET) surface area ABET ≈ 58 m2 g−1, are 5.6 × 10−18 cm2 s−1 and 1.3 × 10−17 cm2 s−1, respectively. Even if we use one-third of total BET surface as an effective area corresponding to the [010] planes,28,29 these diffusion coefficients are found to be in the order of 1.2 × 10−16 cm2 s−1 and 5 × 10−17 cm2 s−1. For LFP-NT the diffusion coefficients are 1.7 × 10−14 cm2 s−1 for oxidation and 1.3 × 10−14 cm2 s−1 reduction peaks, which is two to three orders more compared to the diffusion coefficient of LFP-NP cathode.
is shown in Fig. 9c and d for LFP-NP and LFP-NT, respectively. The calculated anodic and cathodic diffusion coefficients of Li for LFP-NP, using Brunauer–Emmett–Teller (BET) surface area ABET ≈ 58 m2 g−1, are 5.6 × 10−18 cm2 s−1 and 1.3 × 10−17 cm2 s−1, respectively. Even if we use one-third of total BET surface as an effective area corresponding to the [010] planes,28,29 these diffusion coefficients are found to be in the order of 1.2 × 10−16 cm2 s−1 and 5 × 10−17 cm2 s−1. For LFP-NT the diffusion coefficients are 1.7 × 10−14 cm2 s−1 for oxidation and 1.3 × 10−14 cm2 s−1 reduction peaks, which is two to three orders more compared to the diffusion coefficient of LFP-NP cathode.
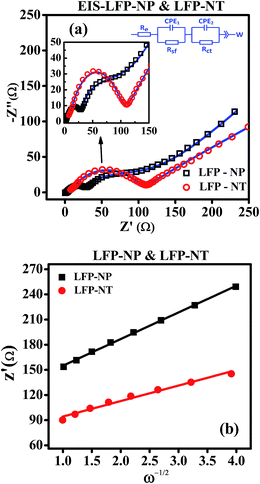
Electrochemical impedance spectroscopy (EIS) is carried out to understand the difference in the electrochemical performance. The EIS data were collected from the same half cells used for charging/discharging and CV measurements. The data were acquired with the cells in the fully discharged condition in the frequency range of 1 MHz to 10 mHz. Fig. 10 shows the Nyquist plots of LFP-NT and LFP-NP samples. The Nyquist plot comprises two semicircles in the high frequency region and a straight line in the low frequency region. The equivalent circuit used to calculate the impedance parameters is shown in the inset of Fig. 10. The impedance spectra can be explained with help of an equivalent circuit which includes solution resistance (Re), an intercept at the (Z′)-axis at high frequency corresponding to the resistance of Li+ conduction in liquid electrolyte, charge-transfer resistance (i.e., Li+ migration resistance) through SEI formed on the cathode surface (Rsf), and the charge transfer resistance (Rct) between the cathode film and electrolyte solution interface. The equivalent circuit also contains two constant phase elements (CPE1 and CPE2) used in place of double layer capacitance. The sloping line in the lower frequency region represents the Warburg impedance (W) corresponding to the Li+ diffusion in the LFP cathode. The charge transfer resistance between the cathode film and current collector is neglected in both samples due to the better electrical connectivity for both electrodes. Impedance spectra of LFP-NT show smaller semicircles compared to LFP-NP indicating the charge transfer resistances are relatively small in the former. The derived impedance parameters are listed in Table 2. The Rct values are 161.2 Ω and 253 Ω for LFP-NT and LFP-NP. All the resistances, i.e., Re, Rct and Rsf, are two to four times smaller for cells made with LFP-NT electrode compared to the half-cells made using LFP-NP cathode.
| Impedance parameters | LFP-NP | LFP-NT |
|---|---|---|
| Re (ohm) | 7.67 | 2.01 |
| Rsf (ohm) | 93.37 | 20.12 |
| Rct (ohm) | 253 | 161.2 |
| Warburg element (W) | 12.93 | 8.51 |
The diffusion coefficient is also obtained from Warburg impedance. The linear response of W as a function of square root of frequency is used to find the Warburg factor (σ). Using following equations, Li-ion diffusion coefficient in the open circuit state was obtained.
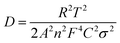 | (2) |
| Z′ = K + σω−1/2 | (3) |
The electrochemical performance of LFP-NT are comparable to previously reported results on various novel microstructures of LiFePO4 including nanoarchitectures, nanosheets, LFP-carbon composites, single crystalline LiFePO4 nanosheets, electrospun nanowires.7,10,16,20,21 To achieve high rate capability and large cyclic stability, the cathode (i) should possess good conductive pathways for Li+ ions and electrons and (ii) be intact as far as the structural and microstructural features are concerned.10 Cathodes made from LFP-NT exhibit good electrical conductivity due to the uniform carbon coating. Though similar carbon layer exists on LFP-NP, Li+ ion insertion and extraction at high current rate is profoundly limited. This is mainly because of the non-accessibility of Li+ ions to the surface of LFP nanoparticles (refer to the schematic in Fig. 11) due to the compact agglomerates present in LFP-NP. This essentially increases the pathway for Li-ion, thereby degrading the rate performance. On the other hand, the open structure of bamboo-like nanotubes in LFP-NT with large contact area between electrolyte and cathode material facilitate easy access of the Li+ ions to the cylindrical surface of nanotubes. The Li diffusion pathways in the olivine structure mainly take place in three directions, viz. [010], [101] and [001] with activation energies for Li-ion migration of 0.55 eV, 2.89 eV and 3.36 eV, respectively.30 The lowest energy barrier for Li diffusion is along b-direction (this forms the 1D-channel for Li diffusion in LFP olivine structure) with a wavelike motion of Li cation.30 Due to the bamboo-like nanotube morphology in LFP-NT, the probability of 1D-channels running across the nanotube axis is large (Fig. 11). Microstructural-openness to such vastly available 1D-channel-entry along b-axis to the electrolyte enables a fast exchange of Li+ ions during intercalation/deintercalation process.31 Well-dispersed and separated nanotubes enable short pathway for Li-ion diffusion further, thereby enhancing the rate performance significantly. Uniform and good quality carbon layers on well crystalline LFP further ensure the stability and long life of LFP-NT cathode in addition to providing the required electrical conductivity for the cathode. The present work emphasizes an important point that the main reason to achieve high-rate capability in LFP system is to have easy access to entry points of 1D channel along the b-axis by creating microstructural-openness during the fabrication process.
In conclusion, bamboo-like LiFePO4 nanotubes with highly oriented single crystalline structures were synthesized by template-assisted method. The electrochemical properties of these LiFePO4 nanotubes are studied in comparison to the LiFePO4 nanoparticles prepared from the same sol–gel precursor solution. Capacity of ∼100 mA h g−1 (∼60 mA h g−1) at a current rate of 10C (25C) with almost 100% capacity retention is demonstrated in LiFePO4 nanotubes when measured for 1000 charging–discharging cycles. Easy access to the entry points of 1D-channels along b-axis is shown to effectively improve the high-rate capability of LiFePO4.
Acknowledgements
C. S. acknowledges the financial support received from the Department of Science and Technology (DST) vide project # SR/S2/CMP-43/2011. C. S. acknowledges the facilities for the cell fabrication and testing through the financial support of Samsung Global Research Outreach (GRO) program.References
- M. S. Whittingham and T. Zawodzinski, Chem. Rev., 2004, 104, 4243–4244 CrossRef CAS PubMed
.
- S.-Y. Chung, J. T. Bloking and Y.-M. Chiang, Nat. Mater., 2002, 1, 123–128 CrossRef CAS PubMed
.
- A. V. Churikov, A. V. Ivanishchev, I. A. Ivanishcheva, V. O. Sycheva, N. R. Khasanova and E. V. Antipov, Electrochim. Acta, 2010, 55, 2939–2950 CrossRef CAS
.
- N. Meethong, Y.-H. Kao, S. A. Speakman and Y.-M. Chiang, Adv. Funct. Mater., 2009, 19, 1060–1070 CrossRef CAS
.
- M. Wagemaker, B. L. Ellis, D. Lützenkirchen-Hecht, F. M. Mulder and L. F. Nazar, Chem. Mater., 2008, 20, 6313–6315 CrossRef CAS
.
- J. Yao, K. Konstantinov, G. X. Wang and H. K. Liu, J. Solid State Electrochem., 2007, 11, 177–185 CrossRef CAS
.
- C. Zhu, Y. Yu, L. Gu, K. Weichert and J. Maier, Angew. Chem., Int. Ed., 2011, 50, 6278–6282 CrossRef CAS PubMed
.
- Y. Xia, M. Yoshio and H. Noguchi, Electrochim. Acta, 2006, 52, 240–245 CrossRef CAS
.
- Y. Wang and G. Cao, Adv. Mater., 2008, 20, 2251–2269 CrossRef CAS
.
- L. Peng, Y. Zhao, Y. Ding and G. Yu, Chem. Commun., 2014, 50, 9569–9572 Search PubMed
.
- C. Delacourt, P. Poizot, S. Levasseur and C. Masquelier, Electrochem. Solid-State Lett., 2006, 9, A352–A355 CrossRef CAS
.
- C. M. Doherty, R. A. Caruso, B. M. Smarsly and C. J. Drummond, Chem. Mater., 2009, 21, 2895–2903 CrossRef CAS
.
- P. Gibot, M. Casas-Cabanas, L. Laffont, S. Levasseur, P. Carlach, S. Hamelet, J.-M. Tarascon and C. Masquelier, Nat. Mater., 2008, 7, 741–747 CrossRef CAS PubMed
.
- S. Lim, C. S. Yoon and J. Cho, Chem. Mater., 2008, 20, 4560–4564 CrossRef CAS
.
- K. Saravanan, M. V. Reddy, P. Balaya, H. Gong, B. V. R. Chowdari and J. J. Vittal, J. Mater. Chem., 2009, 19, 605–610 RSC
.
- C. R. Sides, F. Croce, V. Y. Young, C. R. Martin and B. Scrosati, Electrochem. Solid-State Lett., 2005, 8, A484–A487 CrossRef CAS
.
- J. S. Cho and Y. C. Kang, Small, 2015, 11, 4673–4681 CrossRef CAS PubMed
.
- J. S. Cho, Y. J. Hong and Y. C. Kang, ACS Nano, 2015, 9, 4026–4035 CrossRef CAS PubMed
.
- P. Swain, M. Viji, P. S. V. Mocherla and C. Sudakar, J. Power Sources, 2015, 293, 613–625 CrossRef CAS
.
- Y. Zhao, L. Peng, B. Liu and G. Yu, Nano Lett., 2014, 14, 2849–2853 CrossRef CAS PubMed
.
- X. Rui, X. Zhao, Z. Lu, H. Tan, D. Sim, H. H. Hng, R. Yazami, T. M. Lim and Q. Yan, ACS Nano, 2013, 7, 5637–5646 CrossRef CAS PubMed
.
- F. Bonhomme, J. C. Lassègues and L. Servant, J. Electrochem. Soc., 2001, 148, E450–E458 CrossRef CAS
.
- T. Nakamura, Y. Miwa, M. Tabuchi and Y. Yamada, J. Electrochem. Soc., 2006, 153, A1108–A1114 CrossRef CAS
.
- J. D. Wilcox, M. M. Doeff, M. Marcinek and R. Kostecki, J. Electrochem. Soc., 2007, 154, A389–A395 CrossRef CAS
.
- F. Tuinstra and J. L. Koenig, J. Chem. Phys., 1970, 53, 1126–1130 CrossRef CAS
.
- N. J. Yun, H.-W. Ha, K. H. Jeong, H.-Y. Park and K. Kim, J. Power Sources, 2006, 160, 1361–1368 CrossRef CAS
.
- B. Lung-Hao Hu, F.-Y. Wu, C.-T. Lin, A. N. Khlobystov and L.-J. Li, Nat. Commun., 2013, 4, 1687 CrossRef PubMed
.
- C. Ouyang, S. Shi, Z. Wang, X. Huang and L. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 104303 CrossRef
.
- D. Morgan, A. Van der Ven and G. Ceder, Electrochem. Solid-State Lett., 2004, 7, A30–A32 CrossRef CAS
.
- M. S. Islam, D. J. Driscoll, C. A. J. Fisher and P. R. Slater, Chem. Mater., 2005, 17, 5085–5092 CrossRef CAS
.
- P. Roy and S. K. Srivastava, J. Mater. Chem. A, 2015, 3, 2454–2484 CAS
.
| This journal is © The Royal Society of Chemistry 2016 |