One pot oxidative esterification of benzaldehyde over a supported Cs-salt of mono nickel substituted phosphotungstate†
Anjali
Patel
*,
Soyeb
Pathan
and
Pravya
Prakashan
Polyoxometalates and Catalysis Laboratory, Department of Chemistry, Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara 390002, Gujarat, India. E-mail: aupatel_chem@yahoo.com
First published on 12th May 2016
Abstract
As a cleaner alternative to traditional two steps procedures, the one pot oxidative esterification of benzaldehyde to methyl ester was carried out over a supported Cs salt of mono nickel substituted phosphotungstate (CsPW11Ni). CsPW11Ni was supported on ZrO2 and characterized using various physico-chemical techniques. The influence of reaction parameters, such as molar ratio of substrate to H2O2, amount of catalyst, reaction time, and reaction temperature, on the oxidative esterification reaction was investigated. Moreover, the catalyst could be recovered and reused up to three cycles without significant loss in selectivity. The present heterogeneous catalytic system was found to be efficient not only in terms of activity (63%) but also in selectivity (79%) of the desired product.
Introduction
Esters are valuable chemical products and they have widespread applications such as in the fragrance industry, and as flavoring agents, solvent extractants, diluents and intermediates. The traditional two step synthesis of esters from aldehydes generally suffers from the generation of a vast amount waste, by-products and use of toxic reagents.1 From the viewpoint of demand, as well as the significance of acidic and oxidation reactions, it would be more beneficial to develop bifunctional catalytic systems for single step oxidative esterification reactions. To date, considerable efforts have been devoted to the development of direct synthetic methods for esters from the oxidative esterification of aldehydes with alcohols using different catalysts based on gold-nickel oxide (AuNiOx),2 TS-1,3 gold nanoparticles Au/TiO2,4 Pb and Mg doping in Al2O3-supported Pd,5 the ionic liquid BmimBF4,6 and manganese phthalocyanine immobilized on silica gel.7 In the same context, polyoxometalate (POMs) based materials have been well explored for this reaction.8–10 Recently, a subgroup of POMs, which are transition metal substituted polyoxometalates (TMSPOMs), are gaining remarkable attention in the field of catalysis.11–14 Among the various TMSPOMs ([XW11M(L)O39]5−; X = P, Si; M = transition metal), the nickel-substituted POMs are of considerable interest because of their redox properties and variable oxidation states, which make them important for various catalytic processes.15–17In 2004, Y. Yang et al. prepared TiO2-APS-PW11M (M = Ni/Co) and explored its catalytic performance in organochlorine pesticides and dyes.18 The preparation and detailed characterization of Na5[PW11O39M] (M = Ni2+, Co2+, Cu2+, or Zn2+) supported on carbon was carried out by Pizzio and co-workers in 2007.19 They also evaluated the catalytic activity of the synthesized materials for isopropyl alcohol dehydration. The synthesis and photocatalytic activity of amine-functionalized mesoporous silica/anatase titania impregnated with TMSPOMs ([M(H2O)PW11O39]5−-APS-TiO2, M = Co/Ni) was reported by Guo et al. in 2007.20 Hu and co-workers in 2009, carried out the study of K10−nXn+MW11O39-Schiff-SBA-15, (X = P/Si, M = Co/Ni/Cu/Mn) and its catalytic behaviour was evaluated for the oxidation of styrene to benzaldehyde.21 In 2012, Li et al. prepared MCM-41 incorporated (PW11O39M1)5−(M1–POM, M1 = Ni2+, Co2+ or Cu2+) and its catalytic performance in esterification was investigated.22 Recently, R. A. Frenzel et al. reported the use of transition metal-modified polyoxometalates [PW11O39M(H2O)]5−, where M = Ni2+, Co2+, Cu2+ or Zn2+ supported on carbon as a catalyst in 2-(methylthio)-benzothiazole sulfoxidation.23 Thus, the literature survey shows that there are no reports on the oxidative esterification of aldehyde, which is an important industrial organic transformation, using supported Ni substituted phosphotungstate.
Recently, we reported the one pot oxidative esterification over the Cs salt of mono nickel substituted phosphotungstate (CsPW11Ni), and although the catalyst was homogeneous, it could be recycled upto two cycles without any degradation and modification of its structure and therefore as an extension of that study, we explored heterogeneous behavior of this catalyst by supporting it onto zirconia. As we mentioned in our previous report,24 we have been working on the same catalyst for six months and succeeded to develop a heterogeneous catalyst comprising CsPW11Ni and hydrous zirconia.
Thus, in the present study, we report the synthesis of a Cs salt of mono nickel substituted phosphotungstate supported onto zirconia and its characterization, as well as catalytic activity for oxidative esterification of benzaldehyde to methyl benzoate was evaluated. Different reaction parameters, including percentage loading of catalyst, mole ratio, amount of catalyst, time, temperature, and quantity of methanol, were optimized for better results. The catalyst was recycled and regenerated upto three cycles.
Experimental
Materials
All chemicals used were of A.R. grade. ZrOCl2·8H2O (Loba Chemie), 12-tungstophosphoric acid (H3PW12O40), NaOH, NiCl2·6H2O, CsCl, liq. NH3, C6H5CHO, CH3OH, 30% H2O2, CH2Cl2 were obtained from Merck and used as received.Synthesis of the support, ZrO2
Hydrous zirconia was synthesized using the same method reported by us earlier.25 In the typical procedure, an aqueous ammonia solution was added to an aqueous solution of ZrOCl2·8H2O up to pH 8.5, aged at 100 °C over a water bath for 1 h, filtered, washed with conductivity water until chloride free water was obtained and dried at 100 °C for 10 h. The obtained material is designated as ZrO2.Synthesis of Cs salt of mono nickel substituted phosphotungstate24
H3PW12O40 (2.88 g, 1 mmol) was dissolved in water (10 mL) and the pH of the solution was adjusted to 4.8 using an NaOH solution. NiCl2·6H2O (0.237 g, 1 mmol) dissolved in a minimum amount of water was mixed with the abovementioned hot solution. The final pH was adjusted to 4.8 and heated at 80 °C with stirring for 2 h and filtered hot to which a saturated solution of CsCl was added. The obtained light green crystals were filtered, air dried and designated as CsPW11Ni.Synthesis of Cs salt of mono nickel substituted phosphotungstate supported on zirconia
A series of catalysts containing 10–40% of CsPW11Ni supported on ZrO2 was synthesized using the impregnation method. ZrO2 (1 g) was impregnated with an aqueous solution of CsPW11Ni (0.1/10–0.4/40 g mL−1 of double distilled water) and dried at 100 °C for 10 h. The resulting materials were designated as 10% PW11Ni/ZrO2, 20% PW11Ni/ZrO2, 30% PW11Ni/ZrO2, and 40% PW11Ni/ZrO2.Characterization
The acidity of the catalyst was determined via n-butyl amine and potentiometric tests. Thermogravimetric analysis (TGA) was carried out using a Mettler Toledo Star SW 7.01 up to 600 °C. Adsorption–desorption isotherms were performed on a Micromeritics ASAP 2010 surface area analyzer at −196 °C. Specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method. Fourier transform infrared (FT-IR) spectroscopy was carried out using KBr pellets on a Perkin Elmer instrument. Fourier transform Raman (FT-Raman) spectra were recorded on an FT-Raman spectrophotometer (Bruker FRA 106). Powder X-ray diffraction (powder XRD) was carried out using a Philips diffractometer (PW-1830). Electron spin resonance (ESR) spectra were obtained on a Varian E-line Century series X-band ESR spectrometer at low temperature and scanned from 2000 to 3200 gauss.Catalyst acidity
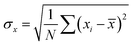 | (1) |
Results and discussion
Characterization of catalyst
The leaching test showed the absence of a blue color, which indicates that there was no leaching of CsPW11Ni from the support into the reaction medium. Furthermore, AAS analysis did not show the presence of any metal content of CsPW11Ni and this suggests the absence of leaching or if any metal was present, it was below the detection limit, which corresponds to less than 1 ppm.The total acidity of the catalysts, 10–40% CsPW11Ni loaded ZrO2, was evaluated via the n-butyl amine titration method and the results are presented in Table 1. It can be observed from Table 1 that with an increase in the percentage loading, the total acidity increases up to 30% loading. Further increase in the percentage loading leads to a decrease in the acidity. A decrease in the acidic value can be attributed to the blocking of acidic sites due to the higher percentage loading.
| Catalyst | Acidic sites mmol of n-butyl amine/g |
|---|---|
| ZrO2 | 0.62 |
| 10% PW11Ni/ZrO2 | 0.68 |
| 20% PW11Ni/ZrO2 | 0.72 |
| 30% PW11Ni/ZrO2 | 0.80 |
| 40% PW11Ni/ZrO2 | 0.75 |
The types and strength of the acidic sites were further obtained via a potentiometric titration method. The strength of the acidic sites in terms of initial electrode potential is shown in Table 2.
| Catalyst | Acidic strength Ei (mV) | Types of acidic sites (meq. g−1) | Total no. of acidic sites | ||
|---|---|---|---|---|---|
| Very strong | Strong | Weak | |||
| ZrO2 | 53 | 0 | 0.5 | 0.8 | 1.3 |
| CsPW11Ni | 50 | 0 | 0.7 | 1.7 | 2.4 |
| 30% PW11/ZrO2 | 58 | 0 | 0.5 | 1.0 | 1.5 |
| 10% PW11Ni/ZrO2 | 55 | 0 | 1.2 | 1.6 | 2.8 |
| 20% PW11Ni/ZrO2 | 70 | 0 | 1.2 | 1.8 | 3.0 |
| 30% PW11Ni/ZrO2 | 110 | 0.2 | 1.3 | 2.2 | 3.7 |
| 40% PW11Ni/ZrO2 | 75 | 0 | 1.4 | 2.5 | 3.9 |
It can be observed from the Table 2 that the incorporation of CsPW11Ni increases the strength of the acidic sites of the catalysts to a great extent. It is interesting to note that 30% PW11Ni/ZrO2 shows the highest acidic strength. For the 40% loaded catalyst the total number of acidic sites is higher, however, acidic strength decreases due to the blocking of the acidic sites at a higher loading. Therefore, 30% PW11Ni/ZrO2 was selected for detailed characterization studies.
The TGA of 30% PW11Ni/ZrO2 (Fig. 1) shows an initial weight loss of 8.8% upto 180 °C, indicating the loss of adsorbed water molecules. Furthermore, about 2.2% weight loss upto 300 °C was observed due to the loss of water of crystallization. In addition to this, no significant weight loss was observed upto 550 °C, which suggests the high thermal stability of the synthesized catalyst.
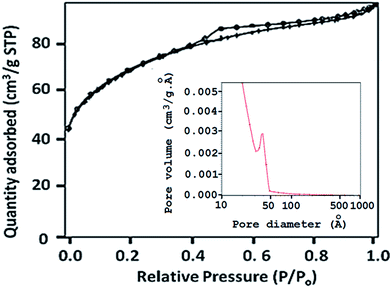
The BET surface area of 30% PW11Ni/ZrO2 was found to be 229 m2 g−1, whereas that of zirconia is 170 m2 g−1.25 The surface area of 30% PW11Ni/ZrO2 was observed to be higher as compared to that of the support zirconia due to the support of PW11Ni, as expected. The pore size distribution curve (Fig. 2) exhibits an average pore diameter of 30.9 Å (3.09 nm).
The FT-IR spectrum of ZrO2 (Table 3) shows broad bands in the region of 3400, 1600 and 1370, and 600 cm−1 which are attributed to O–H asymmetric stretches, H–O–H and O–H–O bending, and Zr–O–H bending, respectively.25 The FT-IR spectrum of CsPW11Ni (Table 3) exhibits bands at 1062; 961; and 885; and 810 cm−1, which correspond to P–O, W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and W–O–W asymmetric stretching frequencies. Moreover, a band at 490 cm−1 is observed, which is attributed to Ni–O vibration, and this indicates the inclusion of the metal ion into the Keggin framework. In the case of CsPW11Ni the splitting value (Δν) for P–O is 0 cm−1, which is in good agreement with the literature.29
O, and W–O–W asymmetric stretching frequencies. Moreover, a band at 490 cm−1 is observed, which is attributed to Ni–O vibration, and this indicates the inclusion of the metal ion into the Keggin framework. In the case of CsPW11Ni the splitting value (Δν) for P–O is 0 cm−1, which is in good agreement with the literature.29
| Catalyst | Frequency (cm−1) | ||||||
|---|---|---|---|---|---|---|---|
| O–H | H–O–H, O–H–O | Zr–O–H | P–O | W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
W–O–W | Ni–O | |
| ZrO2 | 3400 | 1600, 1370 | 600 | — | — | — | — |
| CsPW11Ni | 3447 | 1624 | — | 1062 | 961 | 885, 810 | 490 |
| 30% PW11Ni/ZrO2 | 3255 | 1624, 1375 | 682 | 1064 | 938 | 852 | 453 |
The FT-IR spectrum of 30% PW11Ni/ZrO2 shows similar bands as CsPW11Ni at 1064; 938; 852; and 453; cm−1 which correspond to P–O; W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O; W–O–W and Ni–O, respectively (Fig. 4). In addition, the peaks at 3255; 1624 and 1375; and 682 cm−1 are attributed to O–H stretches, H–O–H and O–H–O bending, and Zr–OH bending, respectively. The shift in the band positions of the supported catalyst as compared to CsPW11Ni is due to the interaction of the terminal oxygen of CsPW11Ni with hydrogen from the surface –OH of zirconia. Thus, it can be observed that the characteristic bands of the active species in the supported catalyst are retained even after the heterogenization.
O; W–O–W and Ni–O, respectively (Fig. 4). In addition, the peaks at 3255; 1624 and 1375; and 682 cm−1 are attributed to O–H stretches, H–O–H and O–H–O bending, and Zr–OH bending, respectively. The shift in the band positions of the supported catalyst as compared to CsPW11Ni is due to the interaction of the terminal oxygen of CsPW11Ni with hydrogen from the surface –OH of zirconia. Thus, it can be observed that the characteristic bands of the active species in the supported catalyst are retained even after the heterogenization.
The Raman spectrum of ZrO2 (Fig. 3) shows broad peaks in the region from 100 to 800 cm−1, which are associated with the long-range disordering arrangement in the amorphous state.30 The Raman spectrum of CsPW11Ni (Fig. 3) shows bands at 993, 976, 895, 506, and 225 cm−1, which correspond to νs (W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Od), νas (W–Od), νas (W–Ob–W), νs (W–Oc–W), and νs (W–Oa), respectively. An additional band in the range of 400–500 cm−1 is attributed to Ni–O stretching and this confirms the presence of nickel in CsPW11Ni. The spectrum of 30% PW11Ni/ZrO2 displays bands at 992, 975, 942, 522, and 213, which correspond to νs (W
Od), νas (W–Od), νas (W–Ob–W), νs (W–Oc–W), and νs (W–Oa), respectively. An additional band in the range of 400–500 cm−1 is attributed to Ni–O stretching and this confirms the presence of nickel in CsPW11Ni. The spectrum of 30% PW11Ni/ZrO2 displays bands at 992, 975, 942, 522, and 213, which correspond to νs (W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Od), νas (W–Od), νas (W–Ob–W), νs (W–Oc–W), and νs (W–Oa), respectively. The presence of all the bands of PW11Ni in 30% PW11Ni/ZrO2 confirms that the structure of CsPW11Ni remains intact even after supporting onto ZrO2. The significant shift observed in the bands is due to the interaction of CsPW11Ni with the surface hydroxyl groups of ZrO2.
Od), νas (W–Od), νas (W–Ob–W), νs (W–Oc–W), and νs (W–Oa), respectively. The presence of all the bands of PW11Ni in 30% PW11Ni/ZrO2 confirms that the structure of CsPW11Ni remains intact even after supporting onto ZrO2. The significant shift observed in the bands is due to the interaction of CsPW11Ni with the surface hydroxyl groups of ZrO2.
The powder XRD pattern of CsPW11Ni is shown in Fig. 4. The peaks ranging from 15° to 30° 2θ indicate that the presence of the characteristic peaks of the parent Keggin ion are shifted in the case of CsPW11Ni due to the substitution of the metal ion into the lacuna. The diffraction peaks corresponding to crystalline phase CsPW11Ni are absent in the XRD pattern of 30% PW11Ni/ZrO2, which confirms the good dispersion of PW11Ni onto the support.
Full range (3200–2000 G) X-band liquid nitrogen temperature ESR spectra for CsPW11Ni (Fig. 5a) and 30% PW11Ni/ZrO2 (Fig. 5b) were also recorded. The obtained g value for CsPW11Ni (g ∼ 2.07) is in good agreement with the reported value,31 which indicates the presence of Ni(II) in an octahedral or distorted octahedral environment. Similarly, the ESR spectra of 30% PW11Ni/ZrO2 shows a signal at g ∼ 2.0, which confirms the presence of undegraded CsPM11Ni on the surface of ZrO2. In other words, the Keggin structure remains unaltered after it was supported on ZrO2.
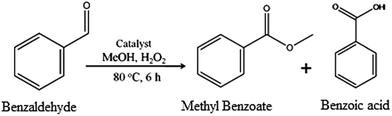
Catalytic activity
To evaluate the efficiency of the catalyst for the direct conversion of aldehydes into their corresponding esters, the reaction of benzaldehyde with methanol in the presence of hydrogen peroxide as an oxidant was carried out, as shown in Scheme 1. The effect of different reaction variables, such as benzaldehyde/H2O2 mole ratio, amount of catalyst, reaction time and temperature, was studied to optimize the conditions for maximum conversion.Effect of percentage loading
The reaction was carried out by varying the percentage loading of the active species (10–40%) onto the support with 10 mg of catalyst for 6 h at 80 °C. The results are presented in Fig. 6, which shows that conversion and selectivity increases with an increase in percentage loading from 10% to 30% PW11Ni/ZrO2. | ||
Fig. 6 Mole ratio (benzaldehyde/H2O2) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), amount of catalyst (10 mg), temperature (80 °C), time (6 h), and volume of methanol (5 mL). 3), amount of catalyst (10 mg), temperature (80 °C), time (6 h), and volume of methanol (5 mL). | ||
However, further increase in loading from 30% to 40% results in a decrease in conversion and selectivity. This may be due to the faster decomposition of H2O2 in the presence of excess active centers in the case of 40% PW11Ni/ZrO2, which results in a decrease in conversion of benzaldehyde. The obtained results may be due to the blocking of acidic sites in the catalyst (Tables 1 and 2). Thus, the 30% PW11Ni/ZrO2 catalyst was selected for detailed catalytic study.
Effect of mole ratio
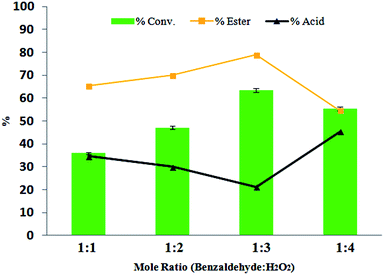
The effect of H2O2 on catalytic activity was studied by varying the mole ratio of benzaldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 from 1
H2O2 from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. As observed in Fig. 7, with an increase in the mole ratio from 1
4. As observed in Fig. 7, with an increase in the mole ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, conversion and selectivity increased simultaneously.
3, conversion and selectivity increased simultaneously.
This increase in conversion may be due to the increase in concentration of H2O2. A decrease in the conversion and selectivity is observed in the case of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Therefore, the 1
4. Therefore, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mole ratio of benzaldehyde
3 mole ratio of benzaldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 was optimized, which gave a 63% conversion and 79% ester.
H2O2 was optimized, which gave a 63% conversion and 79% ester.
Effect of amount of 30% PW11Ni/ZrO2
To determine the optimum amount of catalyst, the reaction was investigated using five different masses of catalyst, keeping the other parameters fixed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mole ratio of benzaldehyde to H2O2 for 6 h at 80 °C and 5 mL of methanol) and the result is presented in Fig. 8.
3 mole ratio of benzaldehyde to H2O2 for 6 h at 80 °C and 5 mL of methanol) and the result is presented in Fig. 8.
 | ||
Fig. 8 Mole ratio (benzaldehyde/H2O2) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), temperature (80 °C), time (6 h), and volume of methanol (5 mL). 3), temperature (80 °C), time (6 h), and volume of methanol (5 mL). | ||
It can be observed from Fig. 8 that the conversion initially increases with an increase in the amount of 30% PW11Ni/ZrO2 from 7.5 mg to 10 mg. However, upon an increase in the catalyst amount from 10 mg, a decrease in conversion, as well as ester selectivity was observed. This decrease in conversion with an increase in the amount of catalyst is due to the rapid unproductive decomposition of H2O2 in the presence of excess amount of catalyst. To confirm the obtained results, the decomposition of H2O2 (SI-1) with different amounts of catalyst was studied and the results are presented in Table S1.† From Table S1,† it is clear that on increasing the catalyst amount, the percentage decomposition of H2O2 increases in the same time. The obtained result suggests that the best utilization of H2O2 was obtained in the case of 10 mg of catalyst, whereas an amount greater than 10 mg results in the unproductive decomposition of H2O2. Moreover, the rapid decomposition of H2O2 generates relatively excess moles of water and this results in decrease in the selectivity of the esterification process, which is a reversible process in presence of water. The reaction carried out using 10 mg of catalyst obtained 63% conversion and 79% ester selectivity, which is quite good with such a minimal amount of catalyst.
Effect of temperature
An increase in temperature resulted in an increase in conversion, as observed in the case of 60 °C and 80 °C. As observed from Fig. 9, at 60 °C good selectivity is observed. In the case of 80 °C, it obtained better conversion and selectivity when compared with that of the other varied temperatures. In the case of 90 °C, conversion and ester selectivity decreased. This may be due to the fast thermal and catalytic decomposition of H2O2 at elevated temperatures. Thus, as a result, 80 °C was selected as the optimum reaction temperature.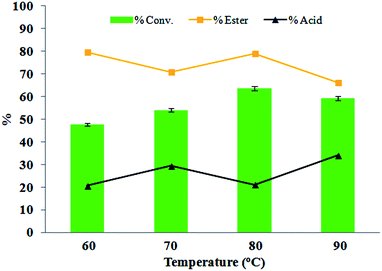 | ||
Fig. 9 Mole ratio (benzaldehyde/H2O2) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), amount of catalyst (10 mg), time (6 h), and volume of methanol (5 mL). 3), amount of catalyst (10 mg), time (6 h), and volume of methanol (5 mL). | ||
Effect of time
For the optimization of reaction time (Fig. 10), the reaction was carried out at various time durations, which resulted in good conversion in the case of 6 h as compared to the other cases. As shown in Fig. 10, with an increase in reaction time, conversion increased proficiently along with selectivity. However, as the reaction time was increased no significant change in conversion and ester selectivity was observed, which is due to attainment of equilibrium in the reaction. As a result, the reaction was optimized for 6 h for better conversion and selectivity.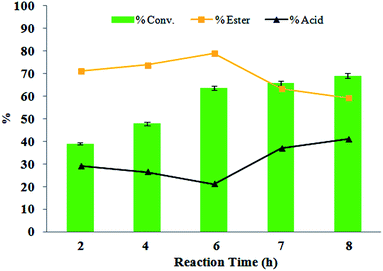 | ||
Fig. 10 Mole ratio (benzaldehyde/H2O2) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), amount of catalyst (10 mg), temperature (80 °C), and volume of methanol (5 mL). 3), amount of catalyst (10 mg), temperature (80 °C), and volume of methanol (5 mL). | ||
Effect of methanol quantity
The effect of methanol volume was also studied for the optimization of the reaction (Table 4). With 2 mL methanol, 67% conversion and 35% ester selectivity was observed.| Methanol quantity | % conv. | % sel. | |
|---|---|---|---|
| Ester | Acid | ||
a Mole ratio benzaldehyde to H2O2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; reaction temperature, 80 °C; and reaction time, 6 h. 3; reaction temperature, 80 °C; and reaction time, 6 h.
|
|||
| 2 mL | 67 | 35 | 65 |
| 5 mL | 63 | 79 | 21 |
| 8 mL | 56.3 | 74.5 | 25.5 |
However, when the volume of methanol was increased to 5 mL, better conversion and selectivity was obtained. Furthermore, it was observed that with an increase in the volume of methanol there was a decrease in conversion, which may be because of the lower availability of hydrogen peroxide for the reaction and dilution of the reaction with an increase in the volume of methanol. Thus, 5 mL methanol was selected as the optimized parameter for the reaction.
Control experiment
The control experiment for the present catalytic system was carried under optimized conditions and results are shown in Table 5. Oxidative esterification of benzaldehyde was carried out without employing any of the catalysts, which obtained a very negligible conversion (3%) and thus proving the need for an efficient catalyst to speed up the reaction. When the supported lacunary counterpart, 30% PW11/ZrO2, was employed as the catalyst, 56.3% conversion with 61.9% ester selectivity was obtained. On comparing this result with the present catalytic system, it is clearly shown that the 30% PW11Ni/ZrO2 is a better catalyst in terms of both activity and selectivity. The control experiment was also carried out with unsupported CsPW11Ni, which shows 59.4% conversion with 62.3% ester selectivity. Furthermore, by comparing the conversion and selectivity for homogeneous, as well as supported systems, it can be observed that the 30% PW11Ni/ZrO2 yields a relatively abundant amount of ester. The obtained data from the control experiments revealed the catalytic activity of the supported catalyst, which is a good source to accelerate and precede the reaction to the desired level. Upon comparison of the activity and selectivity of all the catalysts, 30% PW11Ni/ZrO2 was found to be the best catalyst, because it obtains excellent conversion and selectivity with a very low catalyst amount.Thus, the optimum conditions are mole ratio of benzaldehyde to H2O2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), catalyst amount 10 mg, temperature 80 °C, time 6 h, and methanol volume 5 mL, and the resulting turnover number (TON) is 9690.
3), catalyst amount 10 mg, temperature 80 °C, time 6 h, and methanol volume 5 mL, and the resulting turnover number (TON) is 9690.
Heterogeneity test
Accurate evidence of heterogeneity can be gained only by filtering the catalysts before completion of the reaction and analyzing the filtrate for percentage conversion.32 This test was performed by filtering the catalyst from the reaction mixture at 80 °C after 3 h of the reaction and the filtrate was allowed to react further.The reaction mixture and the filtrate (after 6 h) were analysed via gas chromatography (Table 6). No significant change in the percentage conversion indicates the role of the catalyst in obtaining good yield and selectivity of the desired product. The present catalyst falls into category C,32i.e. the active species does not leach and the observed catalysis is truly heterogeneous in nature.
| Reaction time | % conv. | % ester | % acid |
|---|---|---|---|
| 3 h | 49.8 | 74.5 | 25.4 |
| 6 h | 49.6 | 73.9 | 26.0 |
Regeneration of the catalyst
The catalyst was regenerated to examine its stability and recycled for investigating its activity. The catalyst after the reaction was separated from the reaction mixture by simple centrifugation, washed using dichloromethane and then dried at 100 °C.Characterization of the regenerated catalyst
The regenerated catalyst was further characterized using powder XRD to confirm the retention of the structure of the catalyst.The reused catalyst was characterized via powder XRD, as shown in Fig. 11. There is no appreciable change in the XRD pattern of fresh 30% PW11Ni/ZrO2 and recycled R-30% PW11Ni/ZrO2, which indicates that the material remains unchanged even after regeneration.
Catalytic activity of recycled catalyst
Recycling data (Table 7) shows almost the same conversion for all the catalysts. However, a remarkable decrease in the selectivity of the ester was observed, especially for the first cycle. Subsequently, no significant change in selectivity was observed for subsequent cycles. The observed trend in selectivity can be explained on the basis of the acidity of the catalyst. From the Table 7, it is clearly observed that for the first cycle, the total number of acidic sites decreases considerably as compared to the fresh catalyst, which is responsible for the drastic decrease in selectivity of the ester. However, the total number of acidic sites for the successive recycled catalyst remained constant and as a result no significant change in ester selectivity was observed.| Cycle | Total no. of acidic sites (meq. g−1) | % conv. | % selectivity | Turn over number (TON) | Turn over frequency (TOF) h−1 | |
|---|---|---|---|---|---|---|
| Ester | Acid | |||||
a Reaction conditions: mole ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; reaction temperature, 80 °C; catalyst amount, 10 mg; reaction time, 6 h; and volume of methanol, 5 mL. 3; reaction temperature, 80 °C; catalyst amount, 10 mg; reaction time, 6 h; and volume of methanol, 5 mL.
|
||||||
| Fresh | 3.7 | 63 | 79 | 21 | 9690 | 1615 |
| 1 | 3.2 | 63 | 62 | 38 | 9636 | 1606 |
| 2 | 3.2 | 63 | 64 | 36 | 9587 | 1598 |
| 3 | 3.1 | 61 | 63 | 37 | 9364 | 1560 |
Conclusion
Herein, we report a one pot oxidative esterification for the direct conversion of benzaldehyde to methyl benzoate over a heterogeneous catalyst. The superiority of the catalyst is displayed by its good conversion and excellent selectivity for the methyl benzoate with high TON (9690). The advantages of using a recyclable Ni based catalyst under mild reaction conditions instead of more expensive metals makes this methodology interesting from an economic and an ecological point of view.Acknowledgements
We are thankful to Department of Science and Technology (DST), Project. No. SR/S1/IC-36/2012, New Delhi, for the financial assistance. Ms. Pravya Prakashan is thankful to the same for the award of a research fellowship. We are also thankful to Department of Chemistry, The Maharaja Sayajirao University of Baroda, for N2 adsorption–desorption analysis.References
- J. Otera, Chem. Rev., 1993, 93, 1449–1470 CrossRef CAS.
- K. Suzuki, T. Yamaguchi, K. Matsushita, C. Iitsuka, J. Miura, T. Akaogi and H. Ishida, ACS Catal., 2013, 3, 1845–1849 CrossRef CAS.
- S. P. Chavan, S. W. Dantale, C. A. Govande, M. S. Venkatraman and C. Praveen, Synlett, 2002, 267–268 CrossRef.
- C. Marsden, E. Taarning, D. Hansen, L. Johansen, S. K. Klitgaard, K. Egeblad and C. H. Christensen, Green Chem., 2008, 10, 168–170 RSC.
- Y. Diao, R. Yana, S. Zhang, P. Yang, Z. Li, L. Wang and H. Dong, J. Mol. Catal. A: Chem., 2009, 303, 35–42 CrossRef CAS.
- I. Chiarotto, M. Feroci, G. Sotgiu and A. Inesi, Tetrahedron, 2013, 69, 8088–8095 CrossRef CAS.
- R. K. Sharma and S. Gulati, J. Mol. Catal. A: Chem., 2012, 363–364, 291–303 CrossRef CAS.
- E. Rafiee and S. Eavani, J. Mol. Catal. A: Chem., 2013, 373, 30–37 CrossRef CAS.
- H. Li, Y. Qiao, L. Hua, Z. Hou, B. Feng, Z. Pan, Y. Hu, X. Wang, X. Zhao and Y. Yu, ChemCatChem, 2010, 2, 1165–1170 CrossRef CAS.
- S. Singh and A. Patel, Catal. Lett., 2014, 14, 41557–41567 Search PubMed.
- K. Yamaguchi and N. Mizuno, New J. Chem., 2002, 26, 972–974 RSC.
- M. Faraj and C. L. Hill, J. Chem. Soc., Chem. Commun., 1987, 1487–1489 RSC.
- K. Patel, P. Shringarpure and A. Patel, Transition Met. Chem., 2011, 36, 171–177 CrossRef CAS.
- S. Pathan and A. Patel, Appl. Catal., A, 2013, 459, 59–64 CrossRef CAS.
- Y. Zhanga, H. Lüb, L. Wanga, Y. Zhanga, P. Liua and H. Hana, J. Mol. Catal. A: Chem., 2010, 332, 59–64 CrossRef.
- S.-T. Zheng and G.-Y. Yang, Chem. Soc. Rev., 2012, 41, 7623–7646 RSC.
- S. Mansouri, O. Benlounes, C. Rabia, R. Thouvenot, M. M. Bettahar and S. Hocine, J. Mol. Catal. A: Chem., 2013, 379, 255–262 CrossRef CAS.
- Y. Yang, Y. Guo, C. Hua, Y. Wang and E. Wang, Appl. Catal., A, 2004, 273, 201–210 CrossRef CAS.
- L. R. Pizzio and M. N. Blanco, Mater. Lett., 2007, 61, 719–724 CrossRef CAS.
- Y. Guo and C. Hu, J. Mol. Catal. A: Chem., 2007, 262, 136–148 CrossRef CAS.
- J. Hu, K. Li, W. Li, F. Ma and Y. Guo, Appl. Catal., A, 2009, 364, 211–220 CrossRef CAS.
- B. Li, W. Maa, C. Han, J. Liu, X. Pang and X. Gao, Microporous Mesoporous Mater., 2012, 156, 73–79 CrossRef CAS.
- R. A. Frenzel, G. P. Romanelli, M. N. Blanco and L. R. Pizzio, J. Chem. Sci., 2015, 127, 123–132 CrossRef CAS.
- S. Singh, A. Patel and P. Prakashan, Appl. Catal., A, 2015, 505, 131–140 CrossRef CAS.
- S. Patel, N. Purohit and A. Patel, J. Mol. Catal. A: Chem., 2003, 192, 195–202 CrossRef CAS.
- H. R. Sahu and G. R. Rao, Bull. Mater. Sci., 2000, 23, 349–354 CrossRef CAS.
- P. Vasquez, L. Pizzio, C. Caceres, M. Blanco, H. Thomas and E. Alesso, J. Mol. Catal. A: Chem., 2000, 161, 223–232 CrossRef.
- S. Pathan and A. Patel, Dalton Trans., 2011, 40, 348–355 RSC.
- K. Nowinska, A. Waclaw, W. Masierak and A. Gutsze, Catal. Lett., 2004, 92, 157–162 CrossRef CAS.
- H. Wang, G. Li, Y. Xue and L. Li, J. Solid State Chem., 2007, 180, 2790–2797 CrossRef CAS.
- W. L. Feng, T. H. Chen, L. J. Wang and J. J. Chen, Optik, 2010, 121, 362–365 CrossRef CAS.
- R. A. Sheldon, M. Walau, I. W. C. E. Arends and U. Schuchurdt, Acc. Chem. Res., 1998, 31, 485–493 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04362c |
| This journal is © The Royal Society of Chemistry 2016 |