Synthesis and solution properties of novel thermo- and pH-responsive poly(N-vinylcaprolactam)-based linear–dendritic block copolymers
Gang Tang,
Minqi Hu,
Yongcui Ma,
Dan You and
Yunmei Bi*
College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming 650500, China. E-mail: yunmeibi@hotmail.com
First published on 18th April 2016
Abstract
This study describes the synthesis and solution properties of the novel linear–dendritic block copolymers (LDBCs) based on thermoresponsive poly(N-vinylcaprolactam) (PNVCL) chains and pH-responsive poly(benzyl ether) dendrons. The peripherally methyl ester-functionalized LDBCs (CH3OOC-Gn-b-PNVCL) were first synthesized via atom transfer radical polymerization (ATRP) of N-vinylcaprolactam (NVCL) using first- and second-generation dendritic poly(benzyl ether) chlorides with methyl ester peripheries as initiators. Their peripheral methyl ester groups were then hydrolyzed to afford the target thermoresponsive and pH-responsive poly(N-vinylcaprolactam)-based LDBCs (NaOOC-Gn-b-PNVCL). The results of 1H NMR and GPC analysis showed that the synthesized LDBCs have controlled molecular weight and narrow polydispersity. The results of turbidity and dynamic light scattering (DLS) measurements demonstrated that the lower critical solution temperature (LCST) values of synthesized LDBCs decrease with increase in the generation of the dendritic poly(benzyl ethers) and the concentrations of the LDBCs solutions. The thermoresponsive behavior of NaOOC-Gn-b-PNVCL was also influenced by the pH value of the copolymer solutions. The self-assembly behavior of the LDBCs in an aqueous solution was investigated by fluorescence spectroscopy and transmission electron microscopy (TEM). The results indicated that the morphology of the amphiphilic CH3OOC-Gn-b-PNVCL in an aqueous phase changed from rod-like dendritic structure to a microrectangle shape with increase in the generation of the dendritic poly(benzyl ethers). CH3OOC-Gn-b-PNVCL form more well-defined aggregates. Second critical micelle concentration of NaOOC-G1-b-PNVCL has been observed.
Introduction
There is a growing interest in linear–dendritic block copolymers (LDBCs) as the combination of linear and dendritic polymeric structures in the same macromolecule provides the opportunity to create bulky polymers with easy structural modification and tunable properties.1–3 Among them, amphiphilic LDBCs are particularly attractive due to their potential applications, including drug delivery and enzymatic nanoreactors.4–6 Amphiphilic LDBCs are usually comprises a hydrophilic linear block and hydrophobic dendritic blocks or a hydrophobic linear chain and hydrophilic dendritic blocks. They obtain supramolecular aggregates in solution. In comparison with amphiphilic linear–linear copolymers, the self-assembled aggregates of LDBCs can be manipulated by adjusting not only the length of the linear chain but also the generation number and peripheral groups of dendritic blocks.3 In addition, stimuli-responsive moieties can be incorporated into amphiphilic LDBCs to produce smart materials that are generally processed as self-assemblies of amphiphilic LDBCs with a morphology that can be controlled by external stimuli such as pH value,7,8 temperature,9,10 light11,12 or enzymes.13,14 Among several stimuli, temperature and pH are frequently used because they are relatively convenient and effective stimuli in many applications. Recently, great attention has been focused on multi-stimuli responsive polymers that are sensitive to two or more stimuli because they might greatly enhance the versatility of the material.15,16 Although many single stimulus responsive LDBCs have been synthesized, to date, the only example of dual-responsive LDBCs was reported by Kataoka, Jang, and co-workers.17 They prepared the first thermo and pH dual-responsive LDBCs by conjugating a thermoresponsive poly(2-isopropyl-2-oxazoline) polymer with pH-dependent poly(benzyl ether) dendrons decorated at the periphery with ionisable carboxylic acid groups by CuAAC click chemistry.Poly(N-vinylcaprolactam) (PNVCL), an uncharged, nontoxic, and biocompatible thermoresponsive polymer, exhibits a phase transition in an aqueous solution at temperatures close to body temperature.18–20 Moreover, unlike poly(N-isopropylacrylamide) (PNIPAM), which is the most widely studied thermoresponsive polymer,21 hydrolysis of PNVCL does not result in toxic amine compounds, making it attractive for biomedical and pharmaceutical applications.22,23 However, NVCL belongs to the family of non-conjugated vinyl monomers. It is well known that controlled radical polymerization (CRP) of non-conjugated monomers is not an easy task, because the propagating radicals of these monomers are very reactive and have a tendency to undergo various side reactions during polymerization.24,25 Until recently, the controlled syntheses of NVCL-based homopolymers and copolymers have been demonstrated by various CRP techniques such as the atom transfer radical polymerization (ATRP),26,27 reversible addition–fragmentation chain transfer (RAFT) polymerization28,29 and organometallic-mediated radical polymerization (OMRP).30,31 However, to the best of our knowledge, no study has reported on thermo and pH dual-responsive linear–dendritic block copolymers containing PNVCL.
In this contribution, we report the synthesis and solution properties of well-defined thermo and pH dual-responsive LDBCs containing PNVCL chains and poly(benzyl ether) dendrons with carboxylate peripheries by growing a PNVCL linear arm from the focal point of poly(benzyl ether) dendrons by ATRP.
Experimental
Materials
N-Vinyl caprolactam (NVCL, 98%, Sigma) was distilled under reduced pressure to remove the inhibitor and restored at 4 °C. CuCl was purified by stirring in acetic acid, washed with ethanol and then dried in a vacuum. 5,5,7,12,12,14-Hexamethyl-1,4,8,11-tetra-azacyclotetradecane (Me6Cyclam) was prepared according to the literature method.32 1,4-Dioxane was refluxed on sodium and distilled from sodium benzophenone. CH3OOC-G1-OH and CH3OOC-G2-OH were synthesized according to the method previously reported.33 Isopropanol was used as received.Characterizations
1H NMR spectra were obtained on a Bruker DRX-500 spectrometer in CDCl3 or D2O. FT-IR spectra were obtained on a Nicolet AVATAR 360 FT-IR spectrometer. GPC analyses were performed on a Waters 2690D separations module and a Waters 2414 refractive index detector (RI) with Styragel HR1 and HR4 columns (Waters) using THF as eluent at 40 °C at a flow rate of 0.3 mL min−1. The system was calibrated with linear polystyrene standards. Transmission electron microscopy (TEM) images were obtained using a JEM-2100 transmission electron microscope operating at an accelerating voltage of 200 kV. For TEM analysis, a drop of micelle solution in water (1 mg mL−1) was placed on a Formvar-coated copper grid at room temperature. DLS measurements were performed with a Zetasizer ZEN 3600 instrument (Malvern, UK) operating at 20–50 °C using a light scattering apparatus equipped with a He–Ne laser. The scattering angle was maintained at 173° (back scattering) and the wavelength in the vacuum was set as 633 nm during the whole experiment. Malvern DTS 6.20 software was used to analyse the data. Each reported measurement was the average of three runs.Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield a white solid (1.6 g, yield: 59%). 1H NMR (CDCl3): δ 3.93 (s, 6H, OCH3), 4.51 (d, 2H, J = 5 Hz, CH2Cl), 5.10 (s, 4H, OCH2), 6.54 (t, 1H, J = 2 Hz, CH), 6.64 (d, 2H, J = 2 Hz, CH), 7.49 (d, J = 5 Hz, 4H, CH), 8.08 (d, J = 10 Hz, 4H, CH).
1) to yield a white solid (1.6 g, yield: 59%). 1H NMR (CDCl3): δ 3.93 (s, 6H, OCH3), 4.51 (d, 2H, J = 5 Hz, CH2Cl), 5.10 (s, 4H, OCH2), 6.54 (t, 1H, J = 2 Hz, CH), 6.64 (d, 2H, J = 2 Hz, CH), 7.49 (d, J = 5 Hz, 4H, CH), 8.08 (d, J = 10 Hz, 4H, CH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1196 (C–O).
O), 1196 (C–O).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3.03–3.50 (br, NCH2), 3.90 (s, OCH3), 4.16–4.82 (br, NCH), 5.04–5.16 (br, OCH2), 6.39–6.49 (br, CH), 7.49 (s, CH), 8.07 (s, CH). IR (KBr, cm−1): 2929, 2855 (C–H), 1632 (C
O), 3.03–3.50 (br, NCH2), 3.90 (s, OCH3), 4.16–4.82 (br, NCH), 5.04–5.16 (br, OCH2), 6.39–6.49 (br, CH), 7.49 (s, CH), 8.07 (s, CH). IR (KBr, cm−1): 2929, 2855 (C–H), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1351 (C–O).
O), 1351 (C–O).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1350 (C–O).
O), 1350 (C–O).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3.00–3.53 (br, NCH2), 4.18–4.79 (br, NCH), 5.05–5.17 (br, OCH2), 6.39–6.48 (br, CH), 7.50 (s, CH), 8.06 (s, CH).
O), 3.00–3.53 (br, NCH2), 4.18–4.79 (br, NCH), 5.05–5.17 (br, OCH2), 6.39–6.48 (br, CH), 7.50 (s, CH), 8.06 (s, CH).Turbidity measurement
The LCST values of LDBCs were determined by temperature-dependent turbidity. The optical transmittance of aqueous or LDBC solutions at various pHs at various temperatures were measured at 500 nm with a UV-Vis spectrometer (CARY UV-50, VARIAN) equipped with a water circulation heating stage. The heating rate was 1 °C per 5 min. The lower critical solution temperature (LCST) was defined as the temperature corresponding to a 1% decrease of transmittance.Determination of critical micelle concentration (CMC)
Pyrene was used as a fluorescence probe to measure the CMC of LDBCs in aqueous media. Aliquots of pyrene solution (5 × 10−5 M in acetone, 5 μL) were added to volumetric flasks and the acetone evaporated. The aqueous solutions of CH3OOC-Gn-b-PNVCL or NaOOC-Gn-b-PNVCL at different concentrations were then added to the flasks to get a final pyrene concentration of 4 × 10−7 M. Fluorescence measurements were conducted with a Hitachi F-4500 luminescence spectrophotometer with a band width of 5 nm for excitation and emission. The emission spectra were recorded from 350 nm to 550 nm with an excitation wavelength of 334 nm, and the I383/I373 ratio values of all spectra were calculated.Results and discussion
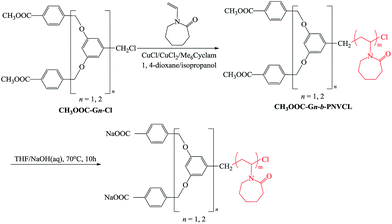
To obtained thermo and pH dual-responsive linear–dendritic block copolymers (LDBCs), we synthesized first linear–dendritic block copolymers (CH3OOC-Gn-b-PNVCL) with linear poly(N-vinylcaprolactam) (PNVCL) blocks and peripherally methyl ester-functionalized Fréchet-type dendrons. Well-defined CH3OOC-Gn-b-PNVCL were synthesized by ATRP of NVCL using two generations of peripherally methyl ester-functionalized Fréchet-type aryl ether dendrons CH3OOC-Gn-Cl as initiators and CuCl/CuCl2/Me6Cyclam as catalyst. The successful synthesis of the desired linear–dendritic block copolymers was confirmed by GPC and 1H NMR spectra. As shown in Fig. 1, the copolymers showed unimodal and symmetrical GPC curves with narrow molecular weight distributions (Mw/Mn ≤ 1.24) without detectable shoulder peaks at high MW positions caused by linear–linear coupling polymers. The 1H NMR spectra of CH3OOC-Gn-b-PNVCL are fully consistent with the proposed chemical structures. For example, the 1H NMR spectrum of CH3OOC-G1-b-PNVCL is shown in Fig. 2a. The peaks at 1.27–1.76, 2.18–2.50 and 3.03–3.50 ppm are assigned to protons k–m, n, and j in the PNVCL segment of the block copolymer, respectively. The peaks at 3.90, 8.07 and 6.39–6.49 ppm are assigned to protons a, b and e(f) in the G1-COOCH3 segment of the block copolymer, respectively.The Mn,NMR values of CH3OOC-Gn-b-PNVCL were determined by comparing the integration ratio of methoxy protons of CH3OOC-Gn at 3.90 ppm and the methylene protons of the PNVCL ring in the α-position to the N atom at 3.03–3.50 ppm. The results are summarized in Table 1. It is shown that the Mn,NMR values of the CH3OOC-Gn-b-PNVCL are in accordance with their Mn,th theoretical values, indicating that they are efficiently prepared and that the ATRP processes were very well controlled. In contrast, the Mn,GPC values of the CH3OOC-Gn-b-PNVCL were quite different from their Mn,th values. This can be attributed to the difference in hydrodynamic volume of the hybrid linear–dendritic block copolymer and the linear standards used for calibration.34–36
| Sample | Mn,tha (g mol−1) | Mn,GPCb (g mol−1) | Mn,NMR (g mol−1) | PDI = Mw/Mn | Conversion (%) |
|---|---|---|---|---|---|
| a The theoretical molecular weight was calculated by the formula: Mn,th = Mmonomer × ([monomer]/[Gn − Cl]) × conversion% + Minitiation.b Mn and PDI were determined by GPC. | |||||
| CH3OOC-G1-b-PNVCL | 9506 | 8505 | 9816 | 1.24 | 65 |
| CH3OOC-G2-b-PNVCL | 9441 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307 307 |
9923 | 1.23 | 60 |
The peripheral methyl ester groups of CH3OOC-Gn-b-PNVCL were hydrolyzed using a 3 M NaOH solution, yielding pH/temperature dual stimuli-responsive linear–dendritic block copolymers (NaOOC-Gn-b-PNVCL). Compared to the 1H NMR spectra of CH3OOC-G1-b-PNVCL (Fig. 2a) and CH3OOC-G2-b-PNVCL (Fig. 3a), the peak at 3.90 ppm corresponding to OCH3 protons disappeared in the 1H NMR spectra of NaOOC-G1-b-PNVCL (Fig. 4a) and NaOOC-G2-b-PNVCL (Fig. 5a), indicating that the terminal methyl ester groups of CH3OOC-Gn-b-PNVCL were completely transformed into the desired carboxylate groups.
The thermally-induced phase transition of the CH3OOC-Gn-b-PNVCL aqueous solutions with various concentrations was investigated by turbidity measurements using UV-Vis spectroscopy. As shown in Fig. 6, the CH3OOC-Gn-b-PNVCL solutions were transparent at room temperature but became very turbid at a specific temperature (LCST). The LCST values of CH3OOC-Gn-b-PNVCL decreased with increasing concentration from 0.04 to 0.80 mg mL−1. In addition, the transition zone became broader upon dilution, which was in correspondence with the thermal phase transition behavior of linear NVCL-based amphiphilic block copolymers.37 It is noteworthy that the transmittance of CH3OOC-Gn-b-PNVCL solution could not decrease to zero at high temperature (above the LCST) when the concentration of the copolymer was lower than 0.4 mg mL−1, similar to the finding of Jérôme.38 This could be attributed to the fact that at low concentrations (below 0.4 mg mL−1), the copolymers are slow in aggregating to a size that can be detected by the spectrophotometer.39,40
 | ||
| Fig. 6 Transmittance measurements as a function of temperature for different concentrations of (a) CH3OOC-G1-b-PNVCL and (b) CH3OOC-G2-b-PNVCL. | ||
CH3OOC-Gn-b-PNVCL exhibited lower LCSTs at all concentrations tested compared to linear PNVCL with a similar MW29 due to the hydrophobicity of the dendritic polyether and its peripheral methyl ester groups. The LCST values of CH3OOC-G2-b-PNVCL are lower than those of CH3OOC-G1-b-PNVCL at all concentrations tested, demonstrating the dependence of LCSTs on the generation of linear–dendritic block polymers. The reason arises from the CH3OOC-G2 dendron possessing more hydrophobic phenyl and peripheral methyl ester groups, which would enhance the hydrophobicity of CH3OOC-G2-b-PNVCL and thus result in a lower LCST.
The thermoresponsive behavior of NaOOC-G1-b-PNVCL and NaOOC-G2-b-PNVCL in deionized water and phosphate-buffered saline (PBS) of varying pH at a concentration of 1 mg mL−1 was also examined by turbidity measurements (Fig. 7 and 8). The LCST values of NaOOC-Gn-b-PNVCL are higher than those of CH3OOC-Gn-b-PNVCL with the same generation. Furthermore, the transition is sharp and the remaining transmittance at high temperature is rather low (a few percent) for NaOOC-G1-b-PNVCL, but for NaOOC-G2-b-PNVCL, the transmittance curve differs markedly in that not only is the transition broader, but also the residual transmittance is much larger (∼45%). This is because the NaOOC-G2 dendron possesses more hydrophilic carboxylate groups, which would enhance the hydrophilicity of NaOOC-G2-b-PNVCL and thus result in a less sensitive phase transition.41
 | ||
| Fig. 7 Transmittance measurements as a function of temperature for NaOOC-G1-b-PNVCL and NaOOC-G2-b-PNVCL in deionized water at a concentration of 1 mg mL−1. | ||
 | ||
| Fig. 8 Transmittance measurements as a function of temperature for (a) NaOOC-G1-b-PNVCL and (b) NaOOC-G2-b-PNVCL in PBS of varying pH at a concentration of 1 mg mL−1. | ||
Fig. 8 shows transmittance for NaOOC-G1-b-PNVCL and NaOOC-G2-b-PNVCL dissolved in phosphate-buffered saline (PBS) of varying pH as a function of temperature at a concentration of 1 mg mL−1. When the pH decreased from 6.64 to 4.92, the LCST of NaOOC-G1-b-PNVCL decreased from 31.5 °C to 29.5 °C. This behavior can be ascribed to the protonation of carboxylates. When the pH value becomes lower, more carboxylate groups in the dendrons are prone to be protonated and the polymer becomes less soluble in aqueous media.42,43 Compared with NaOOC-G1-b-PNVCL (Fig. 8a), NaOOC-G2-b-PNVCL (Fig. 8b) shows more drastic changes and more pH dependence in LCST values, possibly because of the greater number of carboxylates on the peripheries of the poly(benzyl ether) dendron.
The thermoresponsive properties of CH3OOC-Gn-b-PNVCL and NaOOC-Gn-b-PNVCL were further confirmed by measuring their hydrodynamic radii (Rh) against temperature using DLS. As shown in Fig. 9, the particle size dramatically increases at 32–38 °C, from below 50 nm to more than 450–1000 nm. The hydrophobic interactions of the shell above the LCST induce micelle aggregation, leading to the formation of micelles with large Rh. In addition, the aggregation process was completely reversible and the aggregates became soluble again below the onset temperature. This is in good agreement with the results from turbidimetry measurement.
CH3OOC-Gn-b-PNVCL and NaOOC-Gn-b-PNVCL, are composed of hydrophilic PNVCL, as well as –COONa and hydrophobic poly(benzyl ether) dendrons, as well as –COOCH3. They can therefore be self-assembled into micelles in an aqueous solution. The micellar structures of CH3OOC-Gn-b-PNVCL are first confirmed by the fluorescence technique using pyrene as a probe. Red shift of pyrene in the emission spectra was observed with increasing concentration of CH3OOC-Gn-b-PNVCL, indicating the formation of micelles because pyrene preferentially partitions from water into the hydrophobic cores of micelles. The intensity ratio of I383/I373 versus the logarithm of concentrations of CH3OOC-G1-b-PNVCL and CH3OOC-G2-b-PNVCL in the pyrene emission spectra are plotted in Fig. 10a and b, respectively. The ratios have no obvious change below a certain concentration, while the ratios increase remarkably above this concentration, suggesting that pyrene molecules are incorporated into the hydrophobic core region upon micellar aggregation. The critical micelle concentration (CMC) was obtained from the intersection of two straight lines: the baseline and the rapidly increasing I383/I373 line. Thus, the CMC of CH3OOC-G1-b-PNVCL and CH3OOC-G2-b-PNVCL were 0.047 and 0.018 mg mL−1, respectively, as calculated from Fig. 10a and b. Compared to CH3OOC-G1-b-PNVCL, CH3OOC-G2-b-PNVCL has a lower CMC due to its higher hydrophobic dendritic composition.
Interestingly, two abrupt increases in the total fluorescent intensity of NaOOC-G1-b-PNVCL were observed with increasing polymer concentrations (Fig. 10c), indicating NaOOC-G1-b-PNVCL has second CMC value. This is similar to the behavior of some traditional surfactants.44,45 The so-called CMC actually refers to the first critical micelle concentration (FCMC) and spherical micelles are formed at this concentration. The second CMC (SCMC) indicates the structural transition from spherical micelles to rod-like ones.46 The CMC values of NaOOC-G1-b-PNVCL and NaOOC-G2-b-PNVCL were 0.123 and 0.032 mg mL−1, respectively. These values are much higher than those of CH3OOC-Gn-b-PNVCL with the same generation. This could be attributed to the fact that the carboxylates on the peripheries of the poly(benzyl ether) dendrons enhance the hydrophilicity of NaOOC-Gn-b-PNVCL and thus result in higher CMC values.
1H NMR was recorded to confirm the LDBCs micelles formation in an aqueous environment. When the 1H NMR experiments of the LDBCs were performed in D2O, the benzyl proton peaks disappeared at 6.5–8.2 ppm (Fig. 2b and 3b), indicating that the core–shell structure of the self-assembled block copolymers was well preserved under an aqueous environment, thereby restricting the motion of benzyl protons within the hydrophobic core. Similar phenomena were also observed in other amphiphilic linear–dendritic block copolymer systems.47,48
Fig. 11 shows typical TEM images of micelles assembled from CH3OOC-G1-b-PNVCL and CH3OOC-G2-b-PNVCL in water. CH3OOC-G1-b-PNVCL aggregated to form rod-like dendritic micelles whose morphology was quite similar to that of amphiphilic linear–dendritic block copolymers consisting of poly(ε-caprolactone) and monomethoxy poly(ethylene glycol).49 It is observed from the TEM images of CH3OOC-G2-b-PNVCL that some unique microrectangle-shaped structures are forming along with spherical micelles. The spherical structures have radii of the order of 39 nm and are dispersed very well, which was attributed to primary micelles with hydrophobic poly(benzyl ether) dendrons and –COOCH3 being the core and PNVCL being the corona. The microrectangle-shaped structures have a wide distribution of lengths and widths in the range of 200–450 nm, which may be ascribed to multimicellar aggregates,50,51 i.e., CH3OOC-G2-b-PNVCL first self-assembled into small spherical micelles and then the small micelles further aggregated into microrectangle-shaped structures. The amplified microrectangle-shaped structures (arrow) in Fig. 12b, clearly indicate that the large micelles are composed of small spherical building units. The secondary micellar aggregates have also been observed in the case of other amphiphilic linear–dendritic block copolymers.52
 | ||
| Fig. 11 TEM images of the formed micelles by (a) CH3OOC-G1-b-PNVCL, (b) CH3OOC-G2-b-PNVCL, (c) NaOOC-G1-b-PNVCL and (d) NaOOC-G2-b-PNVCL. | ||
 | ||
| Fig. 12 Size distributions of the formed micelles by (a) CH3OOC-G1-b-PNVCL, (b) CH3OOC-G2-b-PNVCL, (c) NaOOC-G1-b-PNVCL and (d) NaOOC-G2-b-PNVCL in water at room temperature. | ||
The mean size and size distribution of the micelles were examined further by DLS at room temperature (Fig. 12). CH3OOC-G1-b-PNVCL appears to have a broad, unimodal distribution of hydrodynamic radii centered around 70 nm. In contrast, the size distribution of the micelles from CH3OOC-G2-b-PNVCL is bimodal with a smaller size population (Rh = 11–39 nm) and a larger population (Rh = 46–229 nm). These results are consistent with the TEM images.
The TEM images in Fig. 11 show that NaOOC-G1-b-PNVCL formed spherical micelles and small irregular-shaped aggregates. The spherical micelles are overlapped with one another. It is observed that short rod-like and irregular-shaped micelles coexist in the TEM image of NaOOC-G2-b-PNVCL. In Fig. 12c and d, the DLS diagrams of NaOOC-G1-b-PNVCL and NaOOC-G2-b-PNVCL also show size distribution over two regions: below 13 nm (Rh) and over 25–171 nm (Rh). It can be observed that CH3OOC-Gn-b-PNVCL have higher hydrophobic content and therefore they form more well-defined aggregates. NaOOC-Gn-b-PNVCL with the lower hydrophobic content tends toward ill-defined structures, because its hydrophobic content is not high enough to organize into more well-defined aggregates.53 It is noteworthy that the sample for a regular TEM measurement is usually prepared by deposition of a drop on the TEM grid followed by evaporation. The drying procedure can affect the morphology of the sample.54 A cryo-TEM should be used to characterize the self-assembled structures of amphiphilic LDBCs if the equipment is available.
Conclusions
The first- and second-generation well-defined peripherally methyl ester-functionalized LDBCs (CH3OOC-Gn-b-PNVCL) were synthesized by ATRP of N-vinylcaprolactam (NVCL) using dendritic poly(benzyl ether) chlorides with methyl ester peripheries as initiators. The transformation of the peripheral methyl ester groups of CH3OOC-Gn-b-PNVCL into the carboxylate groups resulted in thermo- and pH-responsive poly(N-vinylcaprolactam)-based LDBCs (NaOOC-Gn-b-PNVCL). The thermoresponsive properties of NaOOC-Gn-b-PNVCL and its precursor (CH3OOC-Gn-b-PNVCL) were studied and compared by turbidity and dynamic light scattering (DLS) measurements and furthermore, their self-assembly behavior was investigated by fluorescence spectroscopy and transmission electron microscopy (TEM). The LCST values of CH3OOC-Gn-b-PNVCL depend on the generation of the dendritic poly(benzyl ethers) and the concentrations of the LDBCs solutions. NaOOC-Gn-b-PNVCL possesses thermo and pH dual-responsive properties. The multiple morphologies of the micelle aggregates, including spheres, rod-like dendritic structures and microrectangle shapes, were observed, with the variety depending on the generation of the dendritic poly(benzyl ethers) and the various peripheral groups of the LDBCs. CH3OOC-Gn-b-PNVCL forms more well-defined aggregates than NaOOC-Gn-b-PNVCL. A second critical micelle concentration of NaOOC-G1-b-PNVCL has been determined.Acknowledgements
The authors gratefully acknowledge support for this study from the National Natural Science Foundation of China (21264017 and 21564017).Notes and references
- I. Gitsov, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5295–5314 CrossRef CAS.
- F. Wurm and H. Frey, Prog. Polym. Sci., 2011, 36, 1–52 CrossRef CAS.
- E. Blasco, M. Piñol and L. Oriol, Macromol. Rapid Commun., 2014, 35, 1090–1115 CrossRef CAS PubMed.
- G. Whitton and E. R. Gillies, J. Polym. Sci., Part A: Polym. Chem., 2014, 53, 148–172 CrossRef.
- C. M. Dong and G. Liu, Polym. Chem., 2013, 4, 46–52 RSC.
- I. Gitsov, J. Hamzik, J. Ryan, A. Simonyan, J. P. Nakas, S. Omori, A. Krastanov, T. Cohen and S. W. Tanenbaum, Biomacromolecules, 2008, 9, 804–811 CrossRef CAS PubMed.
- C. Román, H. R. Fischer and E. W. Meijer, Macromolecule, 1999, 32, 5525–5531 CrossRef.
- E. R. Gillies, T. B. Jonsson and J. M. J. Fréchet, J. Am. Chem. Soc., 2004, 126, 11936–11943 CrossRef CAS PubMed.
- L. Zhu, G. Zhu, M. Li, E. Wang, R. Zhu and X. Qi, Eur. Polym. J., 2002, 38, 2503–2506 CrossRef CAS.
- Z. Ge, S. Luo and S. Liu, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1357–1371 CrossRef CAS.
- Y. L. Lin, H. Y. Chang and Y. J. Sheng, Macromolecules, 2012, 45, 7143–7156 CrossRef CAS.
- E. Blasco, J. del Barrio, M. Piñol, L. Oriol, C. Berges, C. Sánchez and R. Alcalá, Polymer, 2012, 53, 4604–4613 CrossRef CAS.
- A. J. Harnoy, I. Rosenbaum, E. Tirosh, Y. Ebenstein, R. Shaharabani, R. Beck and R. J. Amir, J. Am. Chem. Soc., 2014, 136, 7531–7534 CrossRef CAS PubMed.
- I. Rosenbaum, A. J. Harnoy, E. Tirosh, M. Buzhor, M. Segal, L. Frid, R. Shaharabani, R. Avinery, R. Beck and R. J. Amir, J. Am. Chem. Soc., 2015, 137, 2276–2284 CrossRef CAS PubMed.
- P. Schattling, F. D. Jochuma and P. Theato, Polym. Chem., 2014, 5, 25–36 RSC.
- J. Zhuang, M. R. Gordon, J. Ventura, L. Li and S. Thayumanavan, Chem. Soc. Rev., 2013, 42, 7421–7435 RSC.
- J. H. Kim, E. Lee, J. S. Park, K. Kataoka and W. D. Jang, Chem. Commun., 2012, 48, 3662–3664 RSC.
- J. Liu, A. Debuigne, C. Detrembleur and C. Jérôme, Adv. Healthcare Mater., 2014, 3, 1941–1968 CrossRef CAS PubMed.
- H. Vihola, A. Laukkanen, L. Valtola, H. Tenhu and J. Hirvonen, Biomaterials, 2005, 26, 3055–3064 CrossRef CAS PubMed.
- G. D. García-Olaiz, K. A. Montoya-Villegas, A. Licea-Claverie and N. A. Cortez-Lemus, React. Funct. Polym., 2015, 88, 16–23 CrossRef.
- P. Liu, J. Liang, S. Chen and H. Zhang, RSC Adv., 2014, 4, 49028–49039 RSC.
- J. Ramos, A. Imazb and J. Forcada, Polym. Chem., 2012, 3, 852–856 RSC.
- M. Prabaharan, J. J. Grailer, D. A. Steeber and S. Q. Gong, Macromol. Biosci., 2009, 9, 744–753 CrossRef CAS PubMed.
- X. Liang, V. Kozlovskay, C. P. Cox, Y. Wang, M. Saeed and E. Kharlampieva, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2725–2737 CrossRef CAS.
- K. Nakabayashi and H. Mori, Eur. Polym. J., 2013, 49, 2808–2838 CrossRef CAS.
- P. Singh, A. Srivastava and R. J. Kumar, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1503–1514 CrossRef CAS.
- Y. Yang, G. Tang, M. Hu, L. Shao, J. Li and Y. Bi, Polymer, 2015, 68, 213–220 CrossRef CAS.
- D. Wan, Q. Zhou, H. Pu and G. Yang, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3756–3765 CrossRef CAS.
- L. Shao, M. Hu, L. Chen, L. Xu and Y. Bi, React. Funct. Polym., 2012, 72, 407–413 CrossRef CAS.
- M. Hurtgen, J. Liu, A. Debuigne, C. Jérôme and C. Detrembleur, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 400–408 CrossRef CAS.
- A. Kermagoret, C. A. Fustin, M. Bourguignon, C. Detrembleur, C. Jérôme and A. Debuigne, Polym. Chem., 2013, 4, 2575–2583 RSC.
- R. W. Hay and G. A. Lawrance, J. Chem. Soc., Perkin Trans. 1, 1975,(6), 591–593 RSC.
- C. J. Hawker, K. L. Wooley and J. M. J. Frechet, J. Chem. Soc., Perkin Trans. 1, 1993,(12), 1287–1297 RSC.
- D. L. Patton, P. Taranekar, T. Fulghum and R. Advincula, Macromolecules, 2008, 41, 6703–6713 CrossRef CAS.
- S. M. Peng, Y. Chen, C. Hua and C. M. Dong, Macromolecules, 2008, 42, 104–113 CrossRef.
- E. Blasco, J. L. Serrano, M. Piñol and L. Oriol, Macromolecules, 2013, 46, 5951–5960 CrossRef CAS.
- Y. Yang, J. Li, M. Hu, L. Chen and Y. Bi, J. Polym. Res., 2014, 21, 549 CrossRef.
- J. Liu, C. Detrembleur, M. C. De Pauw-Gillet, S. Mornet, E. Duguet and C. Jérôme, Polym. Chem., 2014, 5, 799–813 RSC.
- S. Shah, A. Pal, R. Gude and S. Devi, Eur. Polym. J., 2010, 46, 958–967 CrossRef CAS.
- C. Boutris, E. G. Chatzi and C. Kiparissides, Polymer, 1997, 38, 2567–2570 CrossRef CAS.
- M. Gao, X. Jia, G. Kuang, Y. Li, D. Liang and Y. Wei, Macromolecules, 2009, 42, 4273–4281 CrossRef CAS.
- X. Jiang, G. Lu, C. Feng, Y. Li and X. Huang, Polym. Chem., 2013, 4, 3876–3884 RSC.
- I. Popescu, A. Prisacaru, D. M. Suflet and G. Fundueanu, Polym. Bull., 2014, 71, 2863–2880 CrossRef CAS.
- G. Porte, Y. Poggi, J. Appell and G. Maret, J. Phys. Chem., 1984, 88, 5713–5720 CrossRef CAS.
- A. P. Romani, A. E. da Hora Machado, N. Hioka, D. Severino, M. S. Baptista, L. Codognoto, M. R. Rodrigues and H. P. M. de Oliveira, J. Fluoresc., 2009, 19, 327–332 CrossRef CAS PubMed.
- Y. Shi, H. Q. Luo and N. B. Li, Spectrochim. Acta, Part A, 2011, 78, 1403–1407 CrossRef PubMed.
- J. Lee, E. C. Cho and K. Cho, J. Controlled Release, 2004, 94, 323–335 CrossRef CAS PubMed.
- Y. Bi, C. Yan, L. Shao, Y. Wang, Y. Ma and G. Tang, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3240–3250 CrossRef CAS.
- R. Cai, R. Li, J. Qian, A. Xie and K. Nie, Mater. Sci. Eng., C, 2013, 33, 2070–2077 CrossRef CAS PubMed.
- Y. Mai, Y. Zhou and D. Yan, Macromolecules, 2005, 38, 8679–8686 CrossRef CAS.
- H. Hong, Y. Mai, Y. Zhou, D. Yan and J. Cui, Macromol. Rapid Commun., 2007, 28, 591–596 CrossRef CAS.
- Y. Chang and C. Kim, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 918–926 CrossRef CAS.
- Y. Milonaki, E. Kaditi, S. Pispas and C. Demetzos, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1226–1237 CrossRef CAS.
- H. Friedrich, P. M. Frederik, G. de With and N. A. J. M. Sommerdijk, Angew. Chem., Int. Ed., 2010, 49, 7850–7858 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |