Synthesis and biological evaluation of novel analogues of batracylin with synthetic amino acids and adenosine: an unexpected effect on centromere segregation in tumor cells through a dual inhibition of topoisomerase IIα and Aurora B†
Abstract
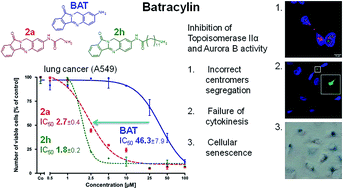
In the search for new anticancer agents we designed and synthesized batracylin derivatives with linking synthetic amino acid side chains of different lengths and adenosine. Unexpectedly, we have found that in water and the culture media adenosine–amino acid–BAT conjugates form supramolecular structures and this prevents these compounds from entering cells. Consequently, these compounds exerted no biological activity when tested towards two human cell lines, lung adenocarcinoma (A549) and human leukemia (HL-60). In contrast, several amino acid–BAT precursors showed up to 25-fold enhanced cytotoxic activity compared to BAT and these compounds strongly interfered with DNA topoisomerase II activity and its cellular functions. In particular, these conjugates inhibited centromere segregation during mitosis in drug-treated tumor cells by preventing topoisomerase II-dependent Aurora B activation.

 Please wait while we load your content...
Please wait while we load your content...